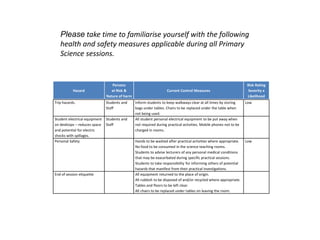
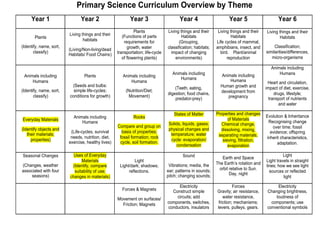
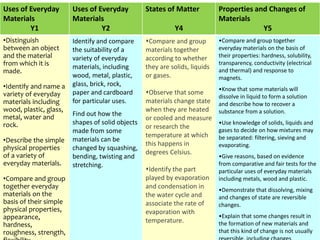
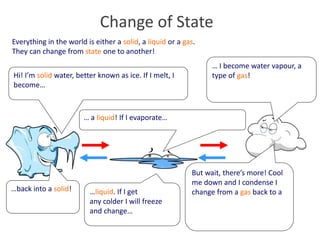
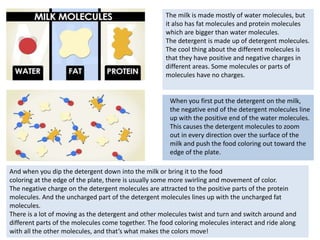
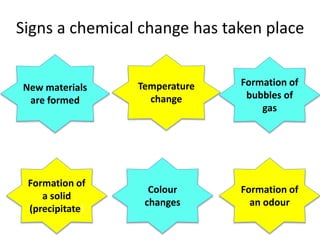
This document provides information for a Primary Science session on materials. It discusses hazards and safety measures, aims of the session including introducing the module and interrogating perceptions of science. It covers scientific enquiry, working scientifically, and different types of material investigations that could be done with students. Examples of curriculum coverage for different year groups related to materials are given, along with potential lesson ideas. An elicitation activity in the form of a subject knowledge quiz on physical and chemical changes is also included. The document aims to prepare teachers for teaching about materials in primary science.