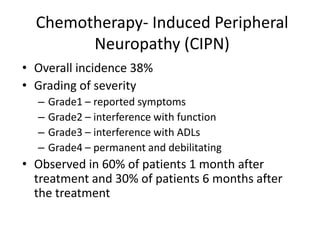
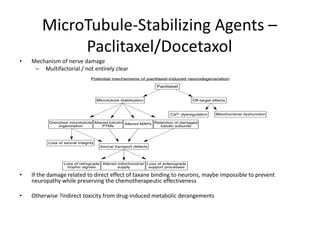
Chemotherapy-induced peripheral neuropathy (CIPN) affects approximately 38% of patients undergoing treatment, particularly with agents like paclitaxel and docetaxel, leading to symptoms that can severely impact daily activities. CIPN can start weeks after chemotherapy begins and may be reversible, though treatment options remain limited and preventative strategies lack solid evidence. Overall, there is no proven association between CIPN and cancer treatment outcomes, highlighting the need for increased focus on prevention and management strategies.