This document summarizes a seminar presentation on the partition coefficient in dyeing textiles. It discusses three key aspects of dye adsorption: (1) transfer of dye molecules to the fiber surface through adsorption, (2) diffusion of dye molecules into the fiber matrix, and (3) displacement of dye molecules into the fiber. It defines the partition coefficient as the ratio of dye concentration in the fiber to concentration in the dye bath solution. Three common dye adsorption isotherm models - Nernst, Freundlich, and Langmuir - are described based on their assumptions about dye site saturation and interaction. Finally, it notes that understanding dyeing mechanisms requires knowledge of thermodynamic and kinetic factors like partition coefficient,

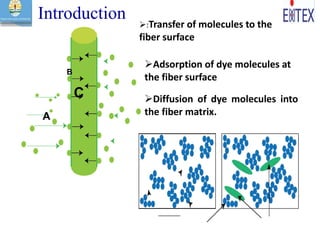
![Dye molecules having diffused
into substrate, displacing fibers
The movement of molecules
from one phase to another is
called partitioning
The partition coefficient, (K), is the partition or distribution of
the dye between the dye bath and fiber phases.
(Partition coefficient) K = 𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑑𝑦𝑒 𝑖𝑛 𝑓𝑖𝑏𝑟𝑒
𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑑𝑦𝑒 𝑖𝑛 𝑠𝑙𝑜𝑢𝑡𝑖𝑜𝑛
It is the ratio of concentration of a dye in fiber phase to the
concentration of a dye in dye bath phase at constant temperature
s
[Df] [[Ds] k= [Df]/[Ds]](https://image.slidesharecdn.com/workuppt-210517115120/85/partition-cofficent-3-320.jpg)
![If two phases are placed adjacent to
each other, the solute/dye will distribute
itself between two phases until
equilibrium is attained;
at equilibrium there is no further
transfer of solute/dye occurs.
The distribution or partition of a solute/dye between two
phases is known as Nernst’s distribution law or simply
distribution law or partition law
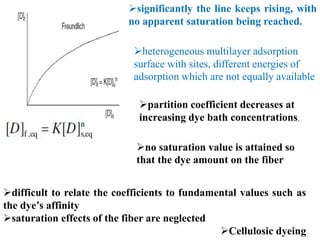
Dye adsorption isotherms are obtained by measuring how much
dye is in the fiber [D]f and how much remains in solution [D]s, when
the dyeing has been allowed to proceed, at a constant temperature,
to equilibrium](https://image.slidesharecdn.com/workuppt-210517115120/85/partition-cofficent-4-320.jpg)
![Three types of adsorption isotherm can be identified, as Nernst,
Freundlich and Langmuir isotherms,
The simplest of the three types
the graph of [D]f plotted against
[D]s is a straight line
The ratio [D]f/[D]s is called the
partition coefficient,
graph is linear, remains constant
until the saturation point of the dye
in the fiber is reached.
no more dye can be taken up by the fiber, no matter how
much more dye is put into the dye bath
Synthetic fiber dyeing (PES)](https://image.slidesharecdn.com/workuppt-210517115120/85/partition-cofficent-5-320.jpg)
![This model is valid for monolayer
sorption onto a surface with a finite
number of identical sites
based on the assumption that
fixed number of dye sites ,one to one
no preferential adsorption at particula
sites
no interaction
limited number of sorption sites, [ D
]sat [mol.kg −1 ], and that the dye takes
place on specific sites in the fiber
dye molecule occupies a site that site is saturated and incapable of
further adsorption
Protein dyeing](https://image.slidesharecdn.com/workuppt-210517115120/85/partition-cofficent-7-320.jpg)


