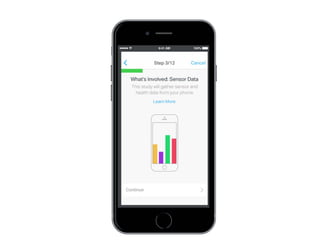
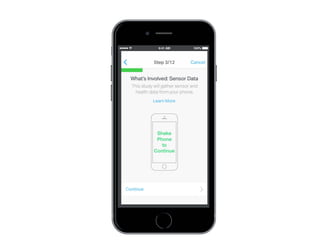
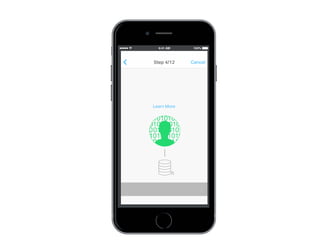
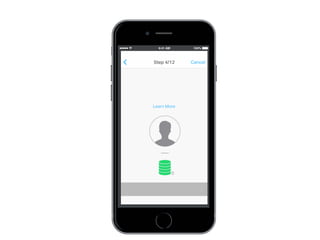
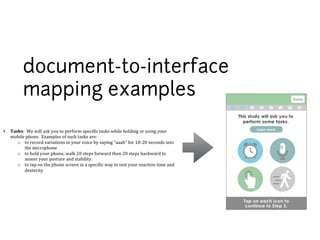
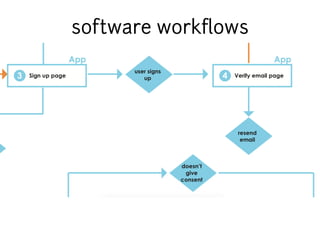
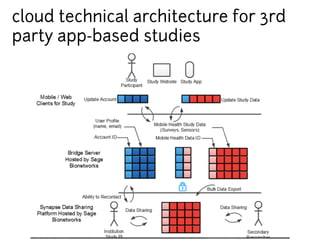
This document discusses the development of a participant-centered consent toolkit by Sage Bionetworks. The toolkit aims to create a user-friendly interface for informed consent documents using visual design tools, templates, and documentation. It includes icons, animations and examples to depict key concepts in clinical studies in an easy-to-understand way. The goal is to deploy consent as a process on mobile and web to better assist participants' understanding of often complex medical studies and research.