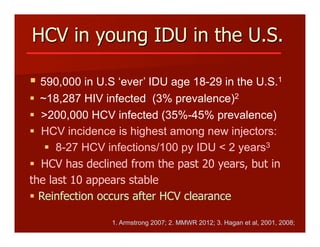
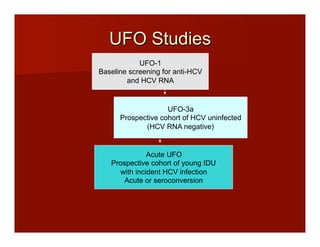
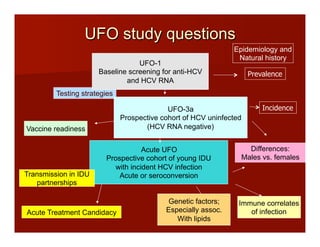
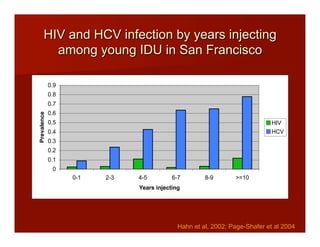
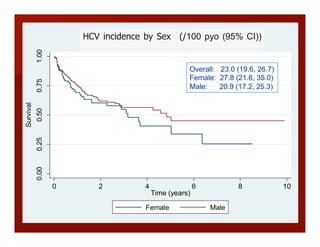
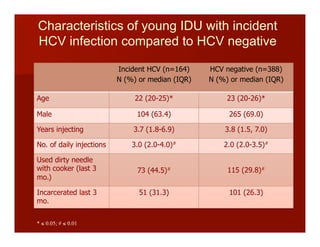
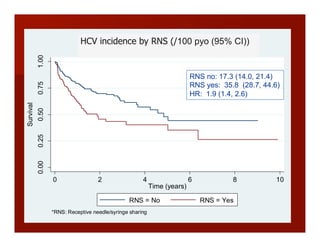
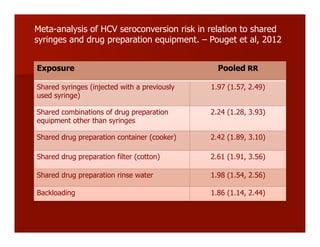
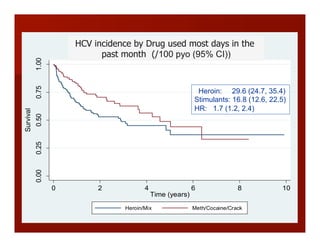
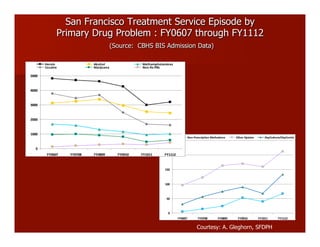
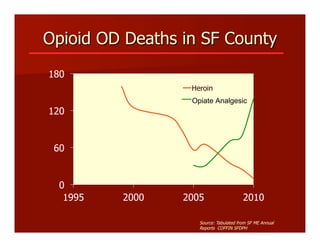
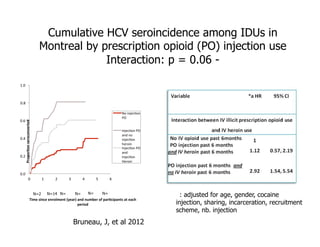
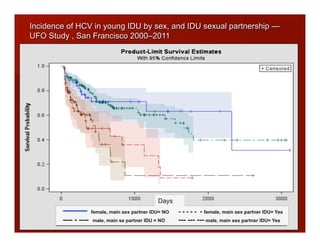
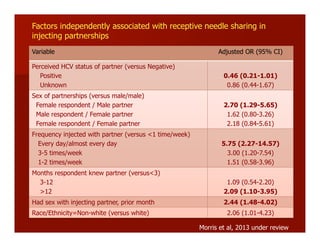
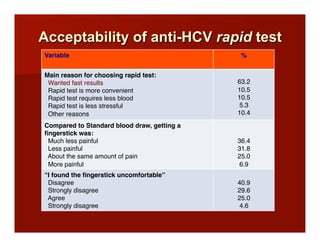
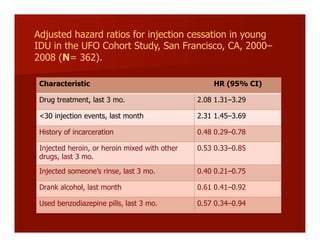
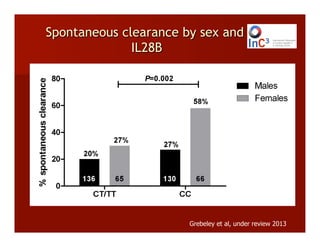
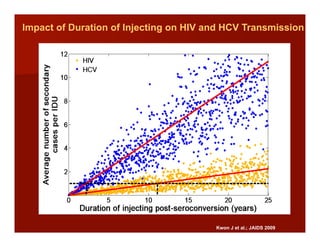
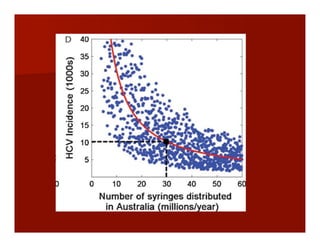
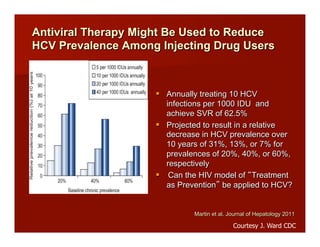
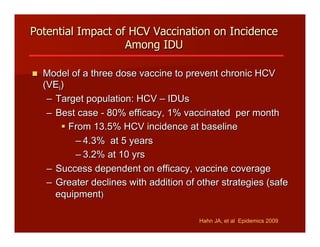
- The document summarizes research on HCV infection in young injection drug users (IDUs) aged 18-29 in the United States. It finds that over 200,000 are estimated to be infected with HCV, with an incidence of 8-27 new infections per 100 person-years of injection drug use for those injecting for less than 2 years. Several studies of young IDUs in San Francisco are described that examine prevalence, incidence, risk factors, partnerships, testing strategies, and spontaneous clearance of HCV. Prevention approaches discussed include increasing HCV treatment rates, developing vaccines, and providing counseling to reduce high-risk behaviors and reinfection.