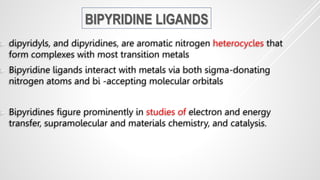
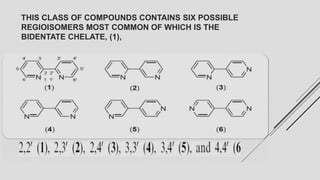
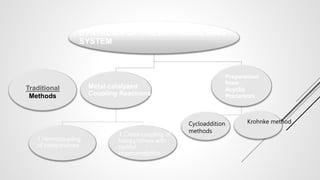
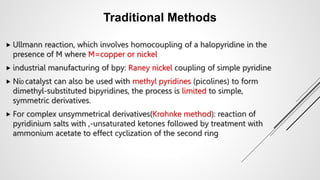
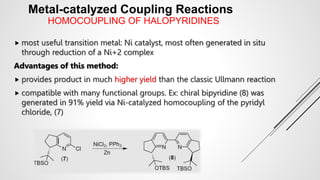
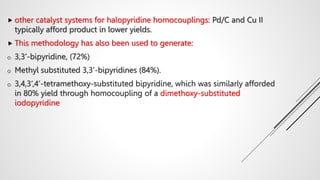
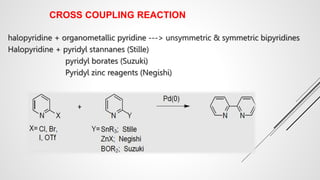
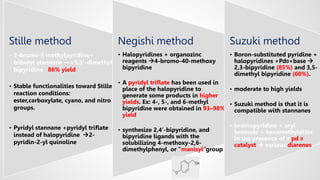
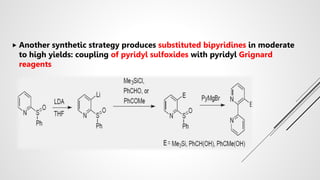
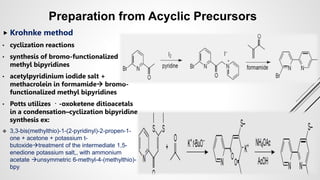
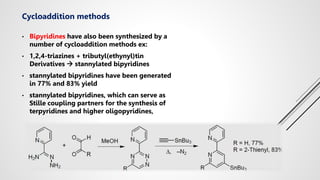
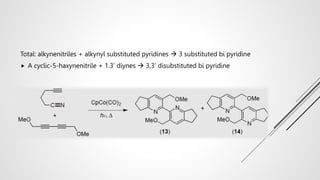
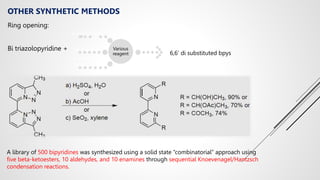
Bipyridine ligands interact with transition metals via their nitrogen atoms and molecular orbitals. There are several methods to synthesize bipyridine rings including traditional coupling reactions, metal-catalyzed coupling reactions, and preparations from acyclic precursors. Metal-catalyzed coupling reactions such as Stille, Suzuki, and Negishi cross-couplings of halopyridines with organometallic pyridines are commonly used to produce unsymmetric and symmetric bipyridines in moderate to high yields. The Krohnke method and cycloaddition reactions are also used to synthesize substituted bipyridine rings from acyclic precursors.