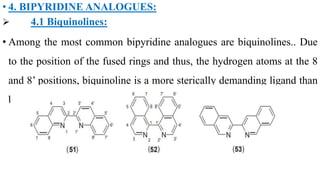
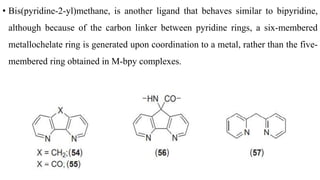
The document discusses various applications and modifications of bipyridine compounds including their use in macrocycles, multidentate chelates, and polymers. It details the synthesis and functionalization of bipyridine with other ligands, metals, and biological molecules, highlighting their versatility in binding and coordination chemistry. Additionally, it explores analogues of bipyridine such as biquinolines and biisoquinolines that serve as ligands in metal coordination.





![•Similar macromolecular bipyridine ligands with large dendritic
wedges in the 4 and 4’ positions have been bound to (RuII)
centers to generate dendrimers with a photoactive [Ru(bpy)3]
2+ core.
•Buckminsterfullerene units have also been incorporated into
bipyridine systems by coupling an acyl chloride-functionalized
C60 molecule with a hydroxy-functionalized bipyridine.
•Two of these ligands were reacted with [Cu(MeCN)4](PF6) to
generate a metal-centered dimer.](https://image.slidesharecdn.com/p4bipyridine-240413153616-ed585ec7/85/Bipyridines-with-Pendant-Macrocycles-The-11-320.jpg)




![ 3.2 Carbohydrates:
The established interaction between boronic acids
and saccharides has been used to selectively
generate and -[Co(bpy)3] 3þ in as high as 79% ee
with þD-glucose.](https://image.slidesharecdn.com/p4bipyridine-240413153616-ed585ec7/85/Bipyridines-with-Pendant-Macrocycles-The-18-320.jpg)