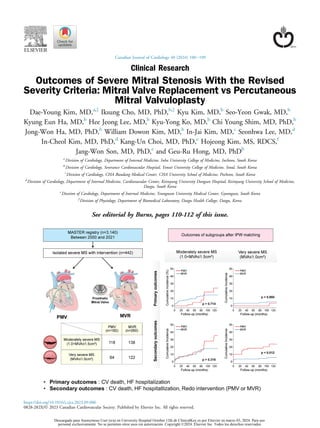
Outcomes of Severe Mitral Stenosis With the Revised Severity Criteria: Mitral Valve Replacement vs Percutaneous Mitral Valvuloplasty
- 1. Clinical Research Outcomes of Severe Mitral Stenosis With the Revised Severity Criteria: Mitral Valve Replacement vs Percutaneous Mitral Valvuloplasty Dae-Young Kim, MD,a,z Iksung Cho, MD, PhD,b,z Kyu Kim, MD,b Seo-Yeon Gwak, MD,b Kyung Eun Ha, MD,b Hee Jeong Lee, MD,b Kyu-Yong Ko, MD,b Chi Young Shim, MD, PhD,b Jong-Won Ha, MD, PhD,b William Dowon Kim, MD,b In-Jai Kim, MD,c Seonhwa Lee, MD,d In-Cheol Kim, MD, PhD,d Kang-Un Choi, MD, PhD,e Hojeong Kim, MS, RDCS,f Jang-Won Son, MD, PhD,e and Geu-Ru Hong, MD, PhDb a Division of Cardiology, Department of Internal Medicine, Inha University College of Medicine, Incheon, South Korea b Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, South Korea c Division of Cardiology, CHA Bundang Medical Center, CHA University School of Medicine, Pocheon, South Korea d Division of Cardiology, Department of Internal Medicine, Cardiovascular Center, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, South Korea e Division of Cardiology, Department of Internal Medicine, Yeungnam University Medical Center, Gyeongsan, South Korea f Division of Physiology, Department of Biomedical Laboratory, Daegu Health College, Daegu, Korea See editorial by Burns, pages 110-112 of this issue. Canadian Journal of Cardiology 40 (2024) 100e109 https://doi.org/10.1016/j.cjca.2023.09.006 0828-282X/Ó 2023 Canadian Cardiovascular Society. Published by Elsevier Inc. All rights reserved. Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
- 2. ABSTRACT Background: This study aimed to compare the outcomes, according to percutaneous mitral valvuloplasty (PMV) vs mitral valve replacement (MVR), of severe mitral stenosis (MS) with the updated criteria (MVA 1.5 cm2 ). Methods: From the Multicenter Mitral Stenosis With Rheumatic Eti- ology (MASTER) registry of 3140 patients, we included patients with severe MS who underwent PMV or MVR between January 2000 and December 2021 except for previous valvular surgery/intervention, at least moderate other valvular dysfunction, and thrombus at the left atrium/appendage. Moderately severe MS (MS-MS) and very severe MS (VS-MS) were defined as 1.0 cm2 MVA 1.5 cm2 and MVA 1.0 cm2 , respectively. Primary outcomes were a composite of cardiovas- cular (CV) death and heart failure (HF) hospitalization. Secondary outcomes were a composite of primary outcomes and redo intervention. Results: Among 442 patients (mean 56.5 11.9 years, women 77.1%), the MVR group (n ¼ 260) was older, had more comorbidities, higher echoscore, larger left chambers, and higher right ventricular systolic pressure than the PMV group (n ¼ 182). During a mean follow- up of 6.9 5.2 years with inverse probability-weighted matching, primary outcomes did not differ, but the MVR group experienced fewer secondary outcomes (P ¼ 0.010). In subgroup analysis of patients with MS-MS and VS-MS, primary outcomes did not differ. However, the MVR group in patients with VS-MS showed better secondary outcomes (P ¼ 0.012). Conclusions: PMV or MVR did not influence CV mortality or HF hos- pitalization in both MS-MS and VS-MS. However, because of increased early redo intervention in the PMV group in VS-MS, MVR would be the preferable option without clear evidence of suitable morphology for PMV. RÉSUMÉ Contexte : La pr esente etude visait à comparer les issues cliniques li ees à la valvuloplastie mitrale percutan ee (VMP) à celles du remplacement de la valve mitrale (RVM) pour corriger la st enose mitrale (SM) s evère, selon les critères les plus r ecents (surface val- vulaire mitrale [SVM] 1,5 cm2 ). M ethodologie : À partir des 3140 patients inscrits au registre multi- centrique Mitral Stenosis With Rheumatic Etiology (MASTER), nous avons inclus dans notre analyse les patients atteints d’une SM s evère ayant subi une VMP ou un RVM entre janvier 2000 et d ecembre 2021, à l’exclusion de ceux ayant auparavant subi une intervention ou une chirurgie valvulaire, qui etaient atteints d’une ou de plusieurs autres dysfonctions valvulaires ou qui pr esentaient une thrombose de l’atrium ou de l’auricule gauche. La SM mod er ee à s evère etait d efinie comme une SVM 1,0 cm2 et 1,5 cm2 , alors que la SM très s evère etait d efinie comme une SVM 1,0 cm2 . Le critère d’ evaluation principal composite comprenait le d ecès d’origine cardiovasculaire (CV) et l’hospitalisation pour insuffisance cardiaque. Le critère d’ evaluation secondaire composite comprenait les el ements du critère principal et la r eintervention. R esultats : Parmi les 442 patients de l’analyse (âge moyen de 56,5 11,9 ans, dont 77,1 % de femmes), les personnes dans le groupe ayant subi une VMP (n ¼ 260) etaient plus âg ees, pr esentaient plus de troubles concomitants et avaient des valeurs plus elev ees pour le score echographique, la taille des cavit es cardiaques gauches et la pression ventriculaire systolique droite que les personnes du groupe ayant subi un RVM (n ¼ 182). Au cours d’un suivi d’une dur ee moyenne de 6,9 5,2 ans, l’analyse avec appariement et pond eration selon la probabilit e inverse n’a montr e aucune diff erence entre les groupes pour le critère principal d’ evaluation, mais la fr equence du critère d’ evaluation secondaire etait plus faible dans le groupe ayant subi un RVM (p ¼ 0,010). L’analyse par sous-groupes des patients pr esentant une SM mod er ee à s evère ou très s evère n’a r ev el e aucune diff erence entre les groupes pour le critère d’ evaluation principal. Chez les patients atteints d’une SM mod er ee à s evère, le groupe ayant subi un RVM a obtenu des r esultats plus favorables pour le critère d’ evaluation secondaire (p ¼ 0,012). Conclusions : Le type d’intervention (VMP ou RVM) n’exerçait pas d’influence sur la mortalit e d’origine CV chez les patients atteints d’une SM mod er ee à s evère ou très s evère. Toutefois, en raison du taux de r eintervention pr ecoce plus elev e dans le groupe de patients atteints d’une SM mod er ee à s evère ayant subi une VMP, il semble que le RVM soit pr ef erable en l’absence d’une morphologie pour laquelle la VMP est manifestement adapt ee. Rheumatic mitral stenosis (MS) remains the most common valvular heart disease worldwide, and despite its decreasing global burden, the condition still has a high prevalence, particularly in the poorest region of the world.1-3 For symp- tomatic severe rheumatic MS, symptoms and prognosis are expected to be improved with optimal surgical or percuta- neous interventional treatment.4 In the current guideline, severe MS is defined as mitral valve (MV) area (MVA) 1.5 cm2 .5-7 In the 2006 American Heart Association guidelines for valvular heart disease, MS severity was divided into moderate and severe, based on the MVA threshold of 1.0 cm2 .8 However, the revised guidelines in 2014 lowered this MVA threshold of severe MS from 1.0 cm2 to 1.5 cm2 ,9 and recently updated guidelines of valvular heart disease still maintained this MS categorization. In the revised guide- lines, identical treatment strategy is recommended for patients with severe MS with MVA 1.0 cm2 and those with MVA of 1.0 to 1.5 cm2 . Without contraindications, percutaneous mitral valvuloplasty (PMV) is recommended as the first-line treatment Received for publication May 31, 2023. Accepted September 9, 2023. z These authors contributed equally to this manuscript. Corresponding author: Dr Geu-Ru Hong, Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Yonsei-ro 50-1, Seodaemun-gu, Seoul, Korea, 03722. E-mail: grhong@yuhs.ac See page 108 for disclosure information. Kim et al. 101 Outcomes of Severe Mitral Stenosis Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
- 3. for severe MS, according to the guidelines. Surgical MV replacement (MVR) is reserved for patients who are ineligible for PMV or require other cardiac surgery. Although this treatment strategy was based on the evidence from several clinical outcome studies,10-13 these results were mostly based on previous criteria of severe MS with MVA 1.0 cm2 , and there are limited data on the clinical outcomes, according to PMV vs MVR treatment strategy, in patients with severe MS, including the moderately severe MS (MS-MS) group (MVA of 1.0 to 1.5 cm2 ) that was recently integrated into the severe grade. Therefore, the purpose of our investigation was to compare the primary outcomesda composite of cardiovascular (CV) death and heart failure (HF) hospitalizationdand the sec- ondary outcomesda composite of primary outcomes and redo interventiondin patients with severe MS undergoing either PMV or MVR, using the updated criteria for the subgroups categorized by MVA of 1.0 cm2 and 1.5 cm2 . Material and Methods Study population We identified 3140 patients with at least moderate MS in the Multicenter Mitral Stenosis With Rheumatic Etiology (MASTER) registry in South Korea between January 2000 and December 2021. First, we screened patients with di- agnoses of severe MS (n ¼ 2652), which was confirmed by transthoracic echocardiography. Severe MS was defined as MVA 1.5 cm2 by 2-dimensional (2D) planimetry according to the latest guidelines for valvular heart disease.5,7 Among them, we excluded patients with previous cardiac surgery or PMV before the indexed echocardiography; combined sig- nificant (at least moderate) mitral regurgitation or aortic ste- nosis/regurgitation; and those with PMV contraindication, including patients having visible thrombus at the left atrium (LA) or LA appendage on transesophageal echocardiography (TEE). After these exclusions, 1325 patients remained as isolated severe MS cases. Among them, 442 patients who experienced therapeutic intervention for MS were finally included in this study (Fig. 1). Treatment strategy was selected by the attending special cardiologists, based on transthoracic echocardiography and TEE, Wilkins echo score, and comorbidity of the patient. All baseline characteristics, echocardiographic parameters, and clinical outcomes of the patients were reviewed retrospec- tively. Indexed echocardiographic data were collected within 3 months before PMV or MVR. The Wilkins score from echocardiographic imaging for each patient was analyzed by the expert cardiologist who was blinded to the clinical outcome. This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board (IRB) of Yonsei University Health System (IRB number: 4-2022-0214). They waived the need for informed consent from patients because of the retrospec- tive nature of this study. Echocardiography Standard 2D and Doppler evaluation were performed in all patients with a standard ultrasound machine in accordance with the guidelines of the American Society of Echocardiography.14 Each cardiac chamber dimension and left ventricular (LV) ejection fraction (EF) was measured by the modified Simpson methods. MVA by 2D planimetry was assessed at the leaflet tips at the mid-diastolic phase.6,15 MVA by pressure half time (PHT) was calculated using the following formula: 220/PHT. The mean diastolic pressure gradient (MDPG) was measured from a continuous wave Doppler signal at both MV leaflet tips. Right ventricular systolic pressure (RVSP) was measured by summing the peak systolic pressure from the maximal tricuspid regurgitation jet velocity, using the modified Bernoulli equation and right atrial pressure, which was estimated by measuring the inferior vena cava diameter. Wilkins echo score was calculated by summing a score from 1 to 4, according to the severity of the mobility, thickness, sub- valvular calcification, and calcification of the morphology of the MV and its apparatus. Regarding the range of severe MS, MS- MS, and very severe (VS)-MS were defined as 1.0 cm2 MVA 1.5 cm2 and MVA 1.0 cm2 , respectively. Follow-up and clinical outcomes After interventional treatment for severe MS, patients regularly visited the outpatient clinic. During follow-up, pa- tients were analyzed for repeated MV intervention, including redo PMV or redo MVR. Basically, all patients were followed up through each hospital’s medical records. For instances in which patients could not be traced within these records, we conducted telephone interviews to ascertain their survival status. In addition, we supplemented our findings by cross- referencing with national mortality data from the Korean Ministry of the Interior and Safety. This allowed us to capture out-of-hospital mortality events and thereby provide more complete data of overall clinical events. Primary outcomes were defined as a composite of cardiovascular death and HF hospitalization. The main secondary outcomes were defined as a composite of cardiovascular death, HF hospitalization, and redo intervention (PMV or MVR). Other outcomes included cardiovascular death, HF hospitalization, ischemic stroke, and systemic embolism. HF hospitalization was defined when the following conditions were met: dyspnea with a New York Heart Association (NYHA) class of at least 3, receiving medical treatment (including intravenous diuretics or vaso- dilator), elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP), and pulmonary congestion or pleural effusion on chest radiography. Ischemic stroke was defined as a focal neurologic deficit of mainly vascular origin without primary cerebral hemorrhage on initial imaging. Systemic embolism was defined as arterial occlusion without evidence of signifi- cant atherosclerosis of the affected artery, except for pulmo- nary embolism and myocardial infarction. If a patient had more than 1 clinical event during follow-up, the first event was counted as the endpoint. Statistical analysis Continuous variables were expressed as mean standard deviation. Categorical variables were expressed as numbers and percentages. We conducted inverse probability-weighted (IPW) analyses to control for known confounders between the two groups undergoing PMV and MVR.16 Initially, propensity scores were computed by using logistic regression that predicts the probability of each treatment option based 102 Canadian Journal of Cardiology Volume 40 2024 Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
- 4. on observed covariates. Subsequently, weights were calculated for each individual as 1/propensity score for the MVR group and 1/ (1 e propensity score) for the PMV group. IPW score was calculated using confounding variables, such as age, sex, atrial fibrillation (AF), hypertension, diabetes mellitus (DM), chronic kidney disease (CKD), MVA by 2D planimetry, LVEF, LV mass index, LA volume index, MDPG, RVSP, and echo score. Kaplan-Meier survival analyses were used to compare the clinical outcomes between 2 treatment groups, and comparison was performed using a log-rank test. For handling of missing data, we used the multiple imputation method to reduce the potential bias arising from each case analysis in this cohort.17 After identifying variables with missing data, multiple imputed datasets by generating plau- sible values were created. Next, we performed the desired analysis individually on each imputed dataset. Finally, the combined results were made from the analyses. P value 0.05 was considered statistically significant. Statistical analyses were performed using R packages (R Foundation for Statistical Computing, Vienna, Austria) and SPSS 25.0 software (IBM Corp, Armonk, New York). Results Baseline clinical characteristics and echocardiographic data A total of 442 patients (mean age: 56.5 11.9 years, women: 77.1%) who underwent PMV or MVR were finally included (MS-MS: n ¼ 256; VS-MS: n ¼ 186). Baseline clinical and echocardiographic characteristics of MS patients according to the treatment strategy (PMV: n ¼ 182; MVR: n ¼ 260) were presented in Table 1. The MVR group was older and had more comorbidities, such as DM, CKD, and AF, than the PMV group. Another comparison of baseline characteristics between patients with MS-MS and VS-MS is shown in Supplemental Table S1. Compared with patients with VS-MS, those with MS-MS were older, had a higher prevalence of DM, and underwent PMV more frequently (compared with MVR) as an initial MS intervention. Patients with MS-MS had lower echo scores, MDPG, and RVSP, and larger LV compared with those who had VS-MS. Further analyses were also performed by comparing treat- ment strategies of PMV and MVR in patients with MS-MS and VS-MS (Table 2). In patients with MS-MS and VS- MS, the MVR group was older and had a higher prevalence of AF. Regarding echocardiographic findings, the MVR group had higher echo scores and larger LA and LV chambers. In patients with MS-MS, the MVR group had a significantly higher prevalence of comorbiditiesdsuch as hypertension, DM, and CKDdand higher MDPG and RVSP. However, these features did not differ significantly between the PMV and MVR groups in patients with VS-MS. Clinical outcomes During a mean follow-up of 6.9 5.2 years, 41 patients experienced primary outcomes, such as cardiovascular death and HF hospitalization, and 63 patients experienced second- ary outcomes (primary outcome and redo intervention). Detailed events are listed in Table 3. As described in Supplemental Fig. S1 and Table 3, the PMV group experi- enced significantly fewer primary outcomes than the MVR group (log-rank, P ¼ 0.030). However, secondary outcomes were not significantly different between 2 groups. Among patients with MS-MS, the PMV group experienced signifi- cantly fewer primary outcomes than the MVR group (log- rank, P ¼ 0.008). However, secondary outcomes were not significantly different between the 2 groups. Among patients with VS-MS, the PMV and MVR groups of VS-MS patients did not differ in their primary outcomes, (log-rank, P ¼ 0.693), but the MVR group had significantly fewer secondary outcomes, with a composite of cardiovascular death, HF hospitalization, and redo intervention, primarily caused by more frequent redo interventions in the PMV group (PMV vs MVR: 10 vs 1). In comparing all-cause death, the outcome Figure 1. Flowchart of the study, illustrating the selection from the Multicenter Mitral Stenosis With Rheumatic Etiology (MASTER) registry. A total of 442 patients with isolated severe MS who underwent PMV or MVR between January 2000 and December 2021 were included. AS, aortic stenosis; AR, aortic regurgitation; LA, left atrium; LAA, left atrial appendage; HF, heart failure; IPW, inverse probability weighting; MR, mitral regurgitation; MS, mitral stenosis; MVA, mitral valve area; MVR, mitral valve replacement; PMV, percutaneous mitral valvuloplasty. Kim et al. 103 Outcomes of Severe Mitral Stenosis Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
- 5. was not significantly different between the 2 groups (Supplemental Fig. S2). Surgical details were additionally analyzed in a total of 260 patients with MVR. More patients had surgery by mechanical valve (n ¼ 180, 69.2%). Most patients received warfarin after MVR (n ¼ 249, 95.8%) than non-vitamin K antagonist oral anticoagulant. In a type of AF, there were more patients of persistent AF (n ¼ 188, 81.7%) (Supplemental Table S2). There were a total of 23 stroke events among the surgical patients. In the results of Kaplan-Meier analysis performed to determine the relationship between valve type and stroke, there was no relationship (P ¼ 0.174) (Supplemental Fig. S3). Additional analysis with IPW methods Given the significant differences in baseline characteristics between the PMV and MVR groups, an IPW-matched sample of the PMV (n ¼ 423.1) and MVR (n ¼ 430.8) groups was generated. As described in Supplemental Table S3, there were no significance differences in clinical and echocardiographic parameters between the PMV and MVR groups of both pa- tients with MS-MS and VS-MS after IPW matching. Kaplan-Meier analyses for outcomes with IPW matching are shown in Figure 2. In the results of all patients with severe MS, there was no significant difference in primary outcomes between the PMV and MVR groups (P ¼ 0.890). The MVR group tended to have more primary outcomes in the early follow-up period, but the PMV group tended to experience late primary outcomes. When redo interventions were included as secondary outcomes, the PMV group experienced significantly more secondary outcomes than the MVR group (log-rank, P ¼ 0.010). In addition, Kaplan-Meier analyses for each outcome, such as all cause of death, redo intervention, and stroke were also performed individually, and the results are shown in Figure 3. Among patients with MS-MS, there were no differences in primary and secondary outcomes between the PMV and MVR groups. Interestingly, the incidence of primary and secondary outcomes in the PMV group rapidly increased after 80 months of follow-up (Fig. 2, B and E). Among patients with VS-MS, there was no difference in primary outcomes between the PMV and MVR groups. However, the MVR group showed better secondary outcomes than the PMV group (log-rank, P ¼ 0.012). Unlike patients with MS-MS, secondary outcomes (mostly redo interventions) in the PMV group of patients with VS-MS occurred from the very early follow-up phase (Fig. 2F). These results implied that MVR immediately after the initial PMV failure played an important role in the poorer prognosis of the PMV group of patients with VS-MS, and it was also confirmed through a separate analysis of the outcome of redo intervention in this group (Fig. 3C). There were no differences, regardless of MVA, between PMV and MVR on the composite outcomes of CV death, HF hospitalization, ischemic stroke, and systemic embolism. In regard to stroke, the chosen treatment strat- egydwhether PMV or MVRddid not influence the occur- rence of outcome (Fig. 3, G-I). Discussion The primary findings of this investigation were as follows: In all patients and each subgroup of patients with MS-MS or VS-MS who underwent PMV or MVR, there was no signif- icance difference in cardiovascular death or admission for HF according to the initial treatment strategy (MVR vs PMV). However, the PMV group experienced more frequent redo interventions in the late (among patients with MS-MS) or early (among patients with VS-MS) follow-up period compared with the MVR group. Therefore, PMV would be considered to be the preferred first-line therapy in patients with MS-MS who have no PMV contraindications and would benefit from deferring open-heart surgery, as suggested by current recommendations. Meanwhile, given the significantly higher rate of early redo interventions in the PMV group compared with the MVR group, MVR would be considered the primary treatment for patients with VS-MS, without clear evidence of suitable morphology for PMV. Previous clinical outcome evaluations of MS-MS and VS-MS Several studies have been undertaken to compare clinical outcomes in patients with severe MS according to treatment options. However, surgical treatment in most previous studies was based on open mitral commissurotomy (OMC) rather than MVR.18 In these studies, PMV and OMC had compa- rable treatment efficacy and safety.10,19 Given the small size of the study population and the low frequency of OMC at present, therapeutic relevance of the outcomes of these in- vestigations is limited. In a more recent study by Song et al., which included MVR in comparison with PMV, patients with MVR had better outcomes than those with PMV.11 This finding is partially consistent with our study results, as it supports the presence of better long-term outcomes of surgical treatment in patients with VS-MS compared with the matched control group with PMV. However, this study used the old criteria of severe MS (MVA 1.0 cm2 ), and the patients in the MVA range of 1.0 cm2 to 1.5 cm2 were not included. Therefore, to our best knowledge, the current study is the first large-scale registry comparison of clinical outcomes, according to MVR and PMV treatment strategy, in patients with severe MS, including those with MVA of 1.0 cm2 to 1.5 cm2 . Distinct characteristics of MS-MS vs VS-MS Although MS-MS is classified under current guidelines in the same severity grade with VS-MS of MVA 1.0 cm2 , its hemodynamic characteristics, as shown in the current study, are different from features traditionally found in the severe grade of MS. In the baseline echocardiography of our study cohort, MDPG, RVSP, and echo score were lower in the MS- MS group than in the VS-MS group. As rheumatic MS progresses, LA pressure and MDPG increase to maintain LV filling, which further triggers pulmonary arteriolar vasocon- striction, leading to an increase in pulmonary artery pres- sure.6,20 RVSP was also associated with severity of disease, and progression of RVSP was associated with poor outcomes in rheumatic patients with MS.21,22 In terms of anatomic fea- tures, the echo score for patients with MS-MS was lower than that for patients with VS-MS in the current study, suggesting that the outcome of PMV would be worse in the VS-MS group than the MS-MS group. Accordingly, MS-MS would 104 Canadian Journal of Cardiology Volume 40 2024 Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
- 6. be considered a distinct group from VS-MS, and a different therapeutic approach could be employed. Treatment strategy in severe MS In our study, patients undergoing PMV were younger, had fewer comorbidities, and showed lower echo scores compared with those in the MVR group. We hypothesize that the PMV group had fewer unfavourable morphologic characteristics, rendering them more suitable candidates for the PMV pro- cedure. In addition, PMV is not considered a definitive treatment that replaces MVR. Because of its inherent limita- tions, it serves primarily as a bridging treatment before surgical intervention. This is why it tends to be chosen for less ris- kydoften youngerdpatients. As such, it is likely our PMV group predominantly consisted of younger and less risky individuals. In this study, there were no differences in primary and secondary outcomes between the PMV and MVR groups of patients with MS-MS. However, the late catch-up phenom- enon caused by the increased prevalence of redointervention in the PMV group was noticeable, particularly after 7 years. This result suggested that PMV mainly serves as a bridging treatment rather than a destination therapy in patients with long life expectancies. Subsequently, PMV would only be considered a destination therapy for very elderly patients who are unable to undergo open-heart surgery or individuals with limited life expectancies. PMV is a procedure that uses a balloon to enlarge a constricted area, rather than replacing the valve completely. It is more comparable with balloon aortic valvuloplasty (BAV)da procedure designed to temporarily widen the area before valve replacementdthan with trans- catheter aortic valve replacement, which involves actual valve Table 1. Baseline clinical and echocardiographic characteristics Total (n ¼ 442) PMV (n ¼ 182) MVR (n ¼ 260) P value Clinical variables Age (year) 56.5 11.9 51.9 12.8 59.7 10.1 0.001 Female sex, n (%) 341 (77.1) 150 (82.4) 191 (73.5) 0.027 BMI, kg/m2 22.9 3.0 22.7 2.8 23.0 3.1 0.265 Hypertension, n (%) 184 (41.6) 66 (36.3) 118 (45.4) 0.056 Diabetes mellitus, n (%) 92 (20.8) 27 (14.8) 65 (25.0) 0.010 CKD, n (%) 20 (4.5) 3 (1.6) 17 (6.5) 0.015 Atrial fibrillation, n (%) 335 (75.8) 105 (57.7) 230 (88.5) 0.001 Follow up duration (y) 6.9 5.2 6.8 5.3 7.0 5.2 0.612 Echocardiography Echoscore 7.7 1.4 7.1 1.3 8.1 1.4 0.001 MVA by 2D, cm2 1.02 0.2 1.05 0.2 1.00 0.2 0.038 LVEF, % 61.0 9.3 62.7 7.9 59.8 10.0 0.001 LA volume index, mL/m2 78.5 36.7 66.8 26.6 86.7 10.5 0.001 LV mass index, mL/m2 84.7 24.3 79.0 19.8 88.6 26.3 0.001 MDPG, mm Hg 8.5 4.3 8.6 3.9 8.4 4.6 0.549 RVSP, mm Hg 40.1 15.7 37.1 13.2 42.2 16.9 0.001 2D, 2-dimensional; BMI, body mass index; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; LA, left atrium; LV, left ventricle; MDPG, mean diastolic pressure gradient of mitral valve; MVA, mitral valve area; MVR, mitral valve replacement; PMV, percutaneous mitral valvuloplasty; RVSP, right ventricular systolic pressure. Table 2. Baseline characteristics of the subgroup according to the MVA difference MS-MS (n ¼ 256) VS-MS (n ¼ 186) PMV (n ¼ 118) MVR (n ¼ 138) P value PMV (n ¼ 64) MVR (n ¼ 122) P value Clinical variables Age (years) 54.3 12.7 61.5 9.0 0.001 47.4 11.8 57.7 11.0 0.001 Female sex, n (%) 95 (80.5) 108 (78.3) 0.774 55 (85.9) 83 (68.0) 0.013 BMI, kg/m2 23.1 2.9 23.6 3.0 0.195 21.9 2.5 22.4 3.0 0.289 Hypertension, n (%) 41 (34.7) 67 (48.6) 0.036 25 (39.1) 51 (41.8) 0.838 Diabetes mellitus, n (%) 21 (17.8) 44 (31.9) 0.015 6 (9.4) 21 (17.2) 0.221 CKD, n (%) 2 (1.7) 11 (8.0) 0.046 1 (1.6) 6 (4.9) 0.425 Atrial fibrillation, n (%) 72 (61.0) 125 (90.6) 0.001 33 (51.6) 105 (86.1) 0.001 NYHA over 3, n (%) 44 (37.3) 63 (45.7) 0.220 19 (29.7) 60 (49.2) 0.016 Follow-up duration (y) 6.8 5.1 6.6 4.9 0.829 6.8 5.8 7.5 5.5 0.419 Echocardiography Echo score 6.9 1.2 7.8 1.4 0.001 7.4 1.2 8.5 1.3 0.001 MVA by 2D, cm2 1.18 0.1 1.19 0.1 0.587 0.81 0.1 0.79 0.2 0.400 LVEF, % 62.0 7.6 60.1 9.6 0.080 64.0 8.2 59.4 10.5 0.001 LA volume index, mL/m2 67.1 28.7 87.6 40.4 0.001 66.322.5 85.6 40.8 0.001 LV mass index, mL/m2 83.7 20.1 93.7 26.2 0.001 70.3 16.2 82.9 25.3 0.001 MDPG, mm Hg 7.2 2.8 6.4 2.8 0.015 11.2 4.3 10.7 5.1 0.422 RVSP, mm Hg 34.0 9.2 38.7 14.2 0.001 42.7 17.2 46.0 18.9 0.228 BMI, body mass index; CKD, chronic kidney disease; EF, ejection fraction; LA, left atrium; LV, left ventricle; MDPG, mean diastolic pressure gradient of mitral valve; MS-MS, moderately severe mitral stenosis; MVA, mitral valve area; MVR, mitral valve replacement; NYHA, New York Heart Association; PMV, percu- taneous mitral valvuloplasty; RVSP, right ventricular systolic pressure; VS-MS, very severe mitral stenosis. Kim et al. 105 Outcomes of Severe Mitral Stenosis Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
- 7. Table 3. Clinical outcomes of the subgroup according to the MVA difference MS-MS (n ¼ 256) VS-MS (n ¼ 186) Total (n ¼ 256) PMV (n ¼ 118) MVR (n ¼ 138) Log-rank P value Total (n ¼ 186) PMV (n ¼ 64) MVR (n ¼ 122) Log-rank P value Primary outcomes 30 7 23 0.008 11 3 8 0.693 Secondary outcomes CV death þ HF þ redo intervention 44 17 27 0.289 19 11 8 0.012 CV death þ HF þ stroke þ embolism 50 17 33 0.056 28 8 20 0.481 CV death 9 5 4 0.543 1 0 1 0.480 HF hospitalization 22 2 20 0.001 10 3 7 0.849 Redo intervention 17 13 4 0.009 11 10 1 0.001 Ischemic stroke 22 11 11 0.701 16 4 12 0.407 Systemic embolism 0 0 0 d* 1 1 0 0.166 CV, cardiovascular; HF, heart failure; MS-MS, moderately severe mitral stenosis; MVA, mitral valve area; MVR, mitral valve replacement; PMV, percutaneous mitral valvuloplasty; VS-MS, very severe mitral stenosis. * Statistical comparison could not be performed because the number of patients was small. Figure 2. Kaplan-Meier analysis of freedom from outcomes based on IPW matching. The MVR group demonstrated superior secondary outcomes (P ¼ 0.010), particularly among patients with VS-MS (P ¼ 0.012), compared with the PMV group. CV, cardiovascular; HF, heart failure. IPW, inverse probability weighting; MVA, mitral valve area; MVR, mitral valve replacement; PMV, percutaneous mitral valvuloplasty; VS-MS, very severe mitral stenosis. 106 Canadian Journal of Cardiology Volume 40 2024 Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
- 8. replacement.23 Both techniques share common challenges such as limited long-term effectiveness, suboptimal valvular function, durability issues, and an associated risk of complications. In patients with VS-MS, there were no differences in pri- mary outcomes between the PMV and MVR groups. How- ever, unlike patients with MS-MS, the MVR group of patients with VS-MS had better secondary outcomes than the PMV group, suggesting that differences in clinical outcomes among patients with VS-MS were primarily attributable to the increased incidence of redo interventions in the PMV group. Redo intervention commonly showed a clear prevalence in the immediate post-PMV period, not in the late phase, as observed in the MS-MS group. This finding implied that redo interventions mainly resulted from unsuccessful PMV or complications of PMV. In previous studies, the increased risk of restenosis and immediate complication after PMV is a factor of important consideration in patients with VS-MS.24-26 As demonstrated in Supplemental Table S3 and Table 2, the VS-MS group had significantly higher echo scores than the MS-MS group. The unfavourable morphology for PMV, including more progressed Figure 3. Kaplan-Meier analysis depicting freedom from specific outcomesddeath, redo intervention, and strokedbased on IPW matching. Among these outcomes, the PMV group exhibited a higher incidence of redo intervention compared with the MVR group, irrespective of the MVA. IPW, inverse probability weighting; MS, mitral stenosis; MVA, mitral valve area; MVR, mitral valve replacement; PMV, percutaneous mitral valvuloplasty. Kim et al. 107 Outcomes of Severe Mitral Stenosis Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
- 9. valvular and subvalvular thickening and commissural calcifica- tion, would be a major contributor to the increased early redointervention rate in the VS-MS group. Further, the assessment system for PMV eligibility is outdated and inaccu- rate for sophisticated MV morphology evaluation.27 Despite the introduction of a few other assessment systems,28 MV morphologic assessment, which determines PMV eligibility, continues to rely primarily on the Wilkins score.29 However, because this score was developed using a very small sample size of only 22 people, it has limitations related to the lack of a detailed location of calcification and leaflet thickening, partic- ularly in the commissural area, which can influence the suc- cessful percutaneous intervention.30 Its clinical applicability in the modern era is quite constrained, and it is necessary to study a model that predicts the possibility of successful percutaneous intervention using other multimodality imaging. Therefore, in patients with VS-MS, given the risk of early redointervention, it is crucial to investigate thoroughly whether there is morphology suitable for attempting PMV; it would only be considered for patients with highly favourable morphology for PMV. Meanwhile, in the current study, early PMV treatment failure was not associated with an increase in cardiovascular death or HF hospitalization (primary out- comes) in the VS-MS group. Therefore, a strategy of an initial PMV, followed by rescue MVR, for patients with PMV failure may be a feasible option for patients with high risk of open heart surgery or those with limited life expectancies. However, as early PMV failure must be carefully considered, a cardiol- ogy team discussion and rigourous morphologic study with the use of multimodal imaging would be beneficial for treatment-plan determination. Study limitations First, this study was retrospectively designed. The possi- bility of differences in clinical outcomes because of the inability to follow patients in the same period could not be excluded. Also, because the patients were included from multicentres based on retrospective nature, and treatment time was quite different for each patient, the consistent standard criteria could not be applied in selecting treatment methods. Second, despite employing IPW matching to adjust for known confounders, the choice of treatment might still be influenced by factors such as patient preference, economic constraints, or physical performance status. This inherent se- lection bias could potentially affect the interpretation of the results. Furthermore, there were still remaining imbalances of both groups despite IPW matching: specifically, in the VS-MS group with age, NYHA, and some echocardiographic vari- ables. Third, we did not include the follow-up echocardio- graphic data related to cardiac symptoms. Therefore, hemodynamic changes of each patient after treatment could not be followed and compared. Conclusions For patients with severe MS, including both MS-MS and VS-MS subgroups, there was no significant difference in cardiovascular mortality or admission for HF according to the initial treatment strategy of PMV vs MVR. However, the PMV group experienced more redo interventions than the MVR group, particularly among patients with VS-MS. Therefore, MVR would be the preferable treatment in pa- tients with VS-MS without clear evidence of suitable morphology for PMV. Ethics Statement The research reported in this manuscript adhered to the institutional ethical guidelines pertaining to historical cohort studies. Patient Consent The authors confirm that patient consent is not applicable to this article. This is a retrospective study using deidentified data; therefore, the IRB did not require consent from the patients. Funding Sources No funding was provided for this paper. Disclosures The authors have no conflicts of interest to disclose. References 1. Rothenbuhler M, O’Sullivan CJ, Stortecky S, et al. Active surveillance for rheumatic heart disease in endemic regions: a systematic review and meta- analysis of prevalence among children and adolescents. Lancet Glob Health 2014;2:e717-26. 2. Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med 2017;377:713-22. 3. Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheu- matic fever and rheumatic heart disease. Clin Epidemiol 2011;3:67-84. 4. Zuhlke L, Engel ME, Karthikeyan G, et al. Characteristics, complica- tions, and gaps in evidence-based interventions in rheumatic heart dis- ease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J 2015;36:1115-1122a. 5. Writing Committee M, Otto CM, Nishimura RA, et al. 2020 ACC/ AHA guideline for the management of patients with valvular heart dis- ease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:e25-197. 6. Silbiger JJ. Advances in rheumatic mitral stenosis: echocardiographic, pathophysiologic, and hemodynamic considerations. J Am Soc Echo- cardiogr 2021;34:709-22. e1. 7. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. 8. Bonow RO, Carabello BA, Kanu C, et al. American College of Cardi- ology, American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guide- lines (writing Committee to Revise the 1998 guidelines for the man- agement of patients with valvular heart disease). J Am Coll Cardiol 2006;48:e1-148. 9. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the 108 Canadian Journal of Cardiology Volume 40 2024 Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
- 10. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. 10. Farhat MB, Ayari M, Maatouk F, et al. Percutaneous balloon versus surgical closed and open mitral commissurotomy: seven-year follow-up results of a randomized trial. Circulation 1998;97:245-50. 11. Song JK, Kim MJ, Yun SC, et al. Long-term outcomes of percutaneous mitral balloon valvuloplasty versus open cardiac surgery. J Thorac Car- diovasc Surg 2010;139:103-10. 12. Kim JB, Ha JW, Kim JS, et al. Comparison of long-term outcome after mitral valve replacement or repeated balloon mitral valvotomy in patients with restenosis after previous balloon valvotomy. Am J Cardiol 2007;99: 1571-4. 13. Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet 2012;379:953-64. 14. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16: 233-70. 15. Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1-23. quiz 101-2. 16. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661-79. 17. Austin PC, White IR, Lee DS, van Buuren S. Missing data in clinical research: a tutorial on multiple imputation. Can J Cardiol 2021;37: 1322-31. 18. Ha JW, Shin WH, Yoon JH, et al. Percutaneous mitral balloon valvu- loplasty in patients with restenosis after surgical commissurotomy: a comparative study. Yonsei Med J 1993;34:243-7. 19. Reyes VP, Raju BS, Wynne J, et al. Percutaneous balloon valvuloplasty compared with open surgical commissurotomy for mitral stenosis. N Engl J Med 1994;331:961-7. 20. Cho IJ, Jeong H, Choi JY, Lee SE, Chang HJ. prognostic implications of the left atrial volume index in patients with progressive mitral stenosis. J Cardiovasc Imaging 2019;27:122-33. 21. Ko KY, Cho I, Kim S, et al. Identification of distinct subgroups in moderately severe rheumatic mitral stenosis using data-driven phenotyping of longitudinal hemodynamic progression. J Am Heart Assoc 2022;11:e026375. 22. Yang B, DeBenedictus C, Watt T, et al. The impact of concomitant pulmonary hypertension on early and late outcomes following surgery for mitral stenosis. J Thorac Cardiovasc Surg 2016;152:394-400.e1. 23. Keeble TR, Khokhar A, Akhtar MM, Mathur A, Weerackody R, Kennon S. Percutaneous balloon aortic valvuloplasty in the era of transcatheter aortic valve implantation: a narrative review. Open Heart 2016;3:e000421. 24. Nunes MCP, Levine RA, Braulio R, et al. Mitral regurgitation after percutaneous mitral valvuloplasty: insights into mechanisms and impact on clinical outcomes. JACC Cardiovasc Imaging 2020;13:2513-26. 25. Meneveau N, Schiele F, Seronde MF, et al. Predictors of event-free survival after percutaneous mitral commissurotomy. Heart 1998;80: 359-64. 26. Kim D, Chung H, Nam JH, et al. Predictors of long-term outcomes of percutaneous mitral valvuloplasty in patients with rheumatic mitral ste- nosis. Yonsei Med J 2018;59:273-8. 27. Nunes MC, Tan TC, Elmariah S, et al. The echo score revisited: impact of incorporating commissural morphology and leaflet displacement to the prediction of outcome for patients undergoing percutaneous mitral val- vuloplasty. Circulation 2014;129:886-95. 28. Farman MT, Khan N, Sial JA, et al. Predictors of successful percutaneous transvenous mitral commissurotomy using the Bonhoeffer multi-track system in patients with moderate to severe mitral stenosis: can we see beyond the Wilkins score? Anatol J Cardiol 2015;15:373-9. 29. Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percu- taneous balloon dilatation of the mitral valve: an analysis of echocar- diographic variables related to outcome and the mechanism of dilatation. Heart 1988;60:299-308. 30. Iung B, Vahanian A. Rheumatic and calcific mitral stenosis and mitral commisurotomy. In: Valvular Heart Disease: A Companion to Braun- wald’s Heart Disease. 5th ed. Philadelphia: Elsevier, 2020:311-36. Supplementary Material To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10. 1016/j.cjca.2023.09.006. Kim et al. 109 Outcomes of Severe Mitral Stenosis Descargado para Anonymous User (n/a) en University Hospital October 12th de ClinicalKey.es por Elsevier en marzo 03, 2024. Para uso personal exclusivamente. No se permiten otros usos sin autorización. Copyright ©2024. Elsevier Inc. Todos los derechos reservados.
