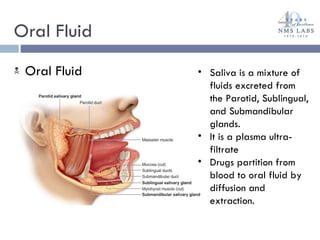
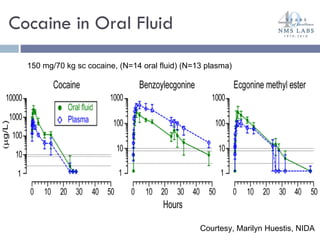
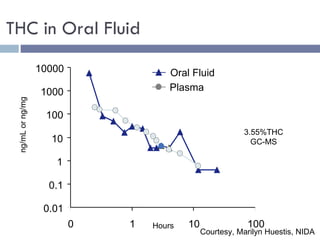
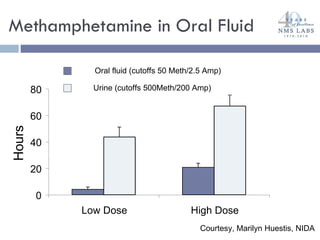
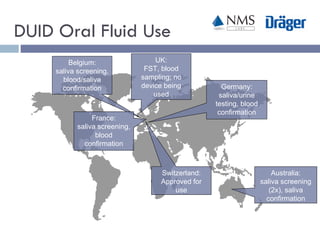
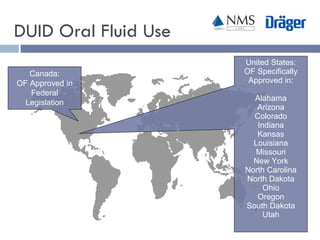
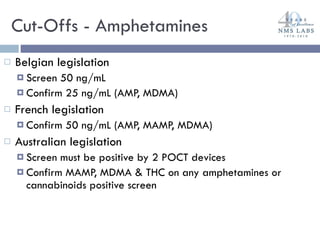
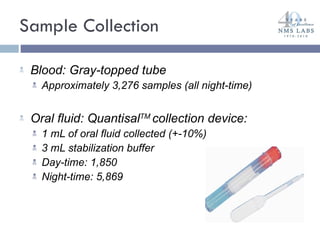
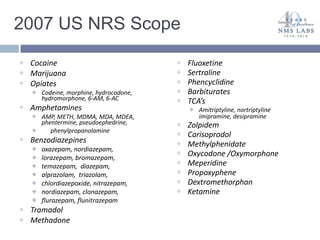
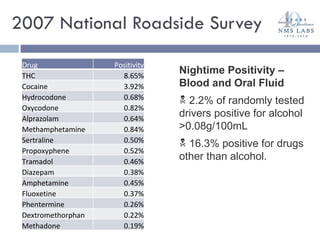
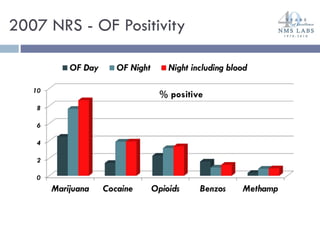
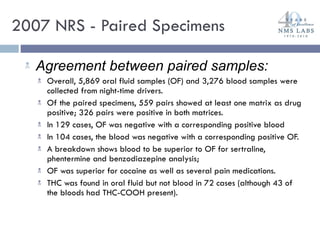
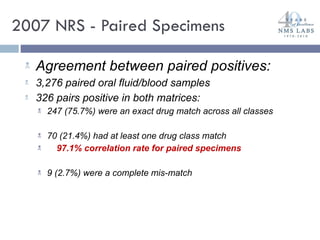
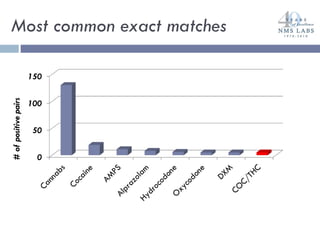
The document discusses the use of oral fluid testing as a method for drug testing within the Drug Recognition Expert (DRE) program, highlighting its advantages such as non-invasive collection and potential for on-site testing. It reviews the history of drug testing, current testing approaches, and the effectiveness of oral fluid testing compared to other methods like urine and blood testing. The document emphasizes that while oral fluid testing is promising, it requires careful planning and validation to ensure accuracy and reliability.