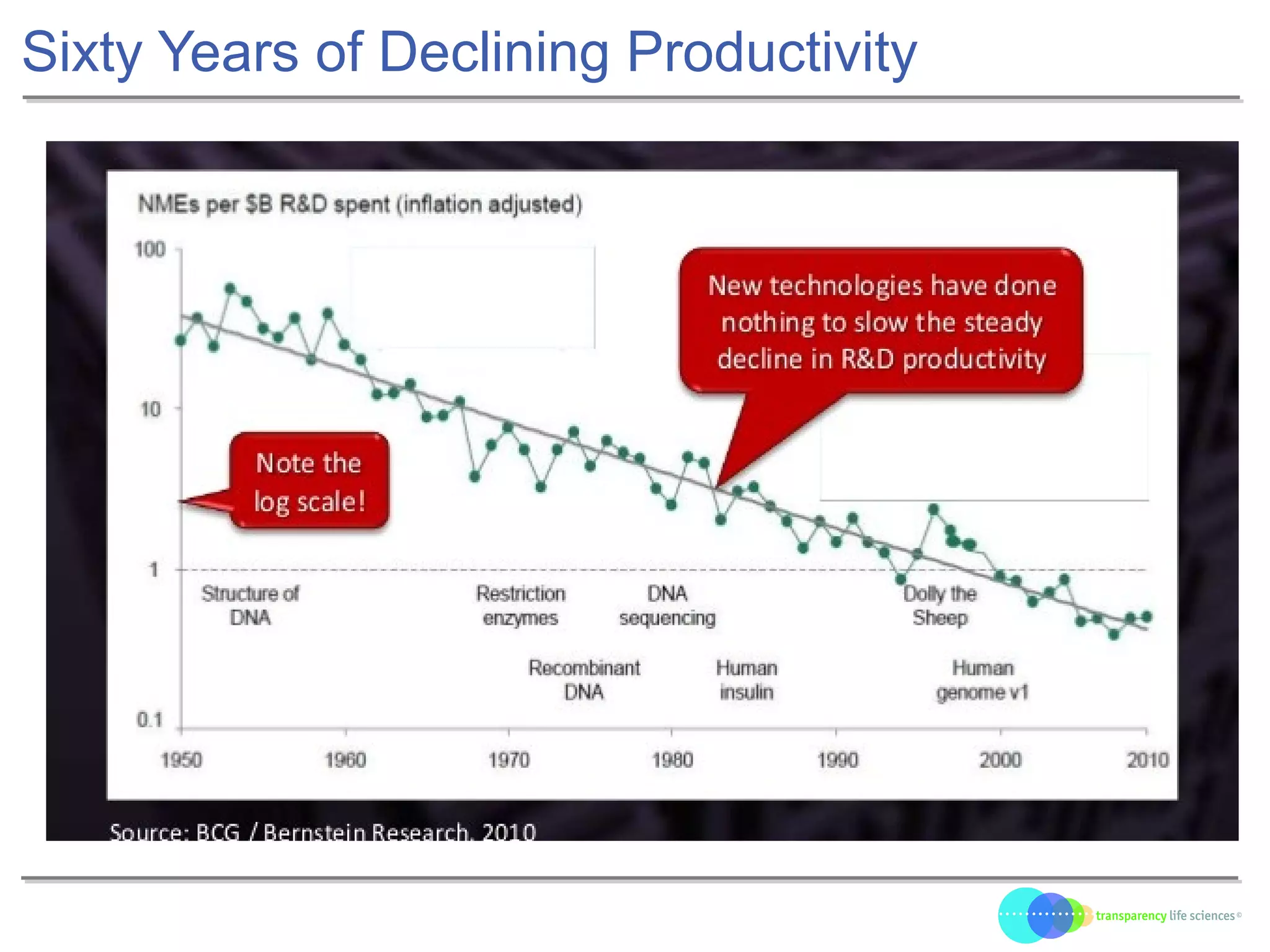
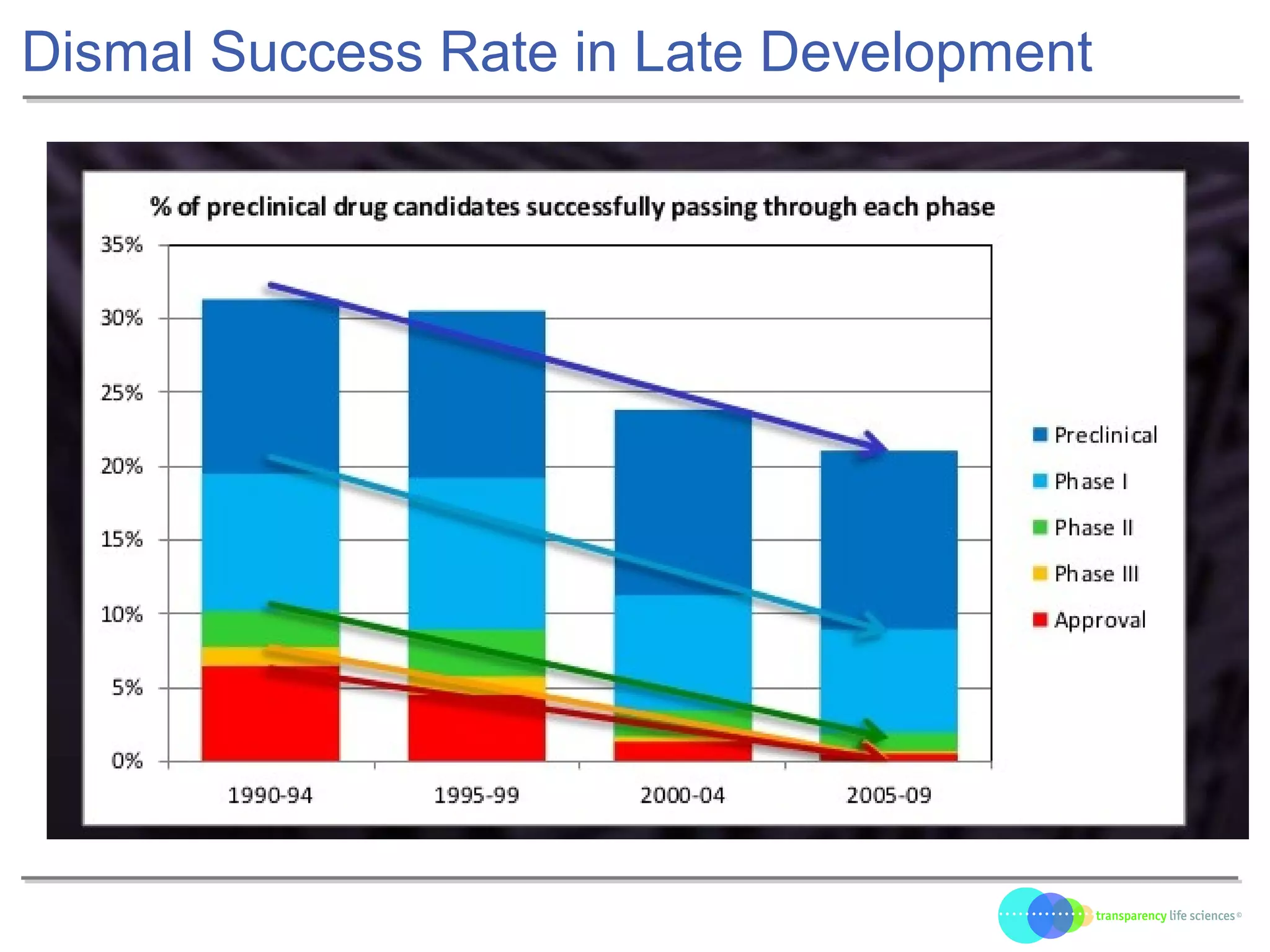
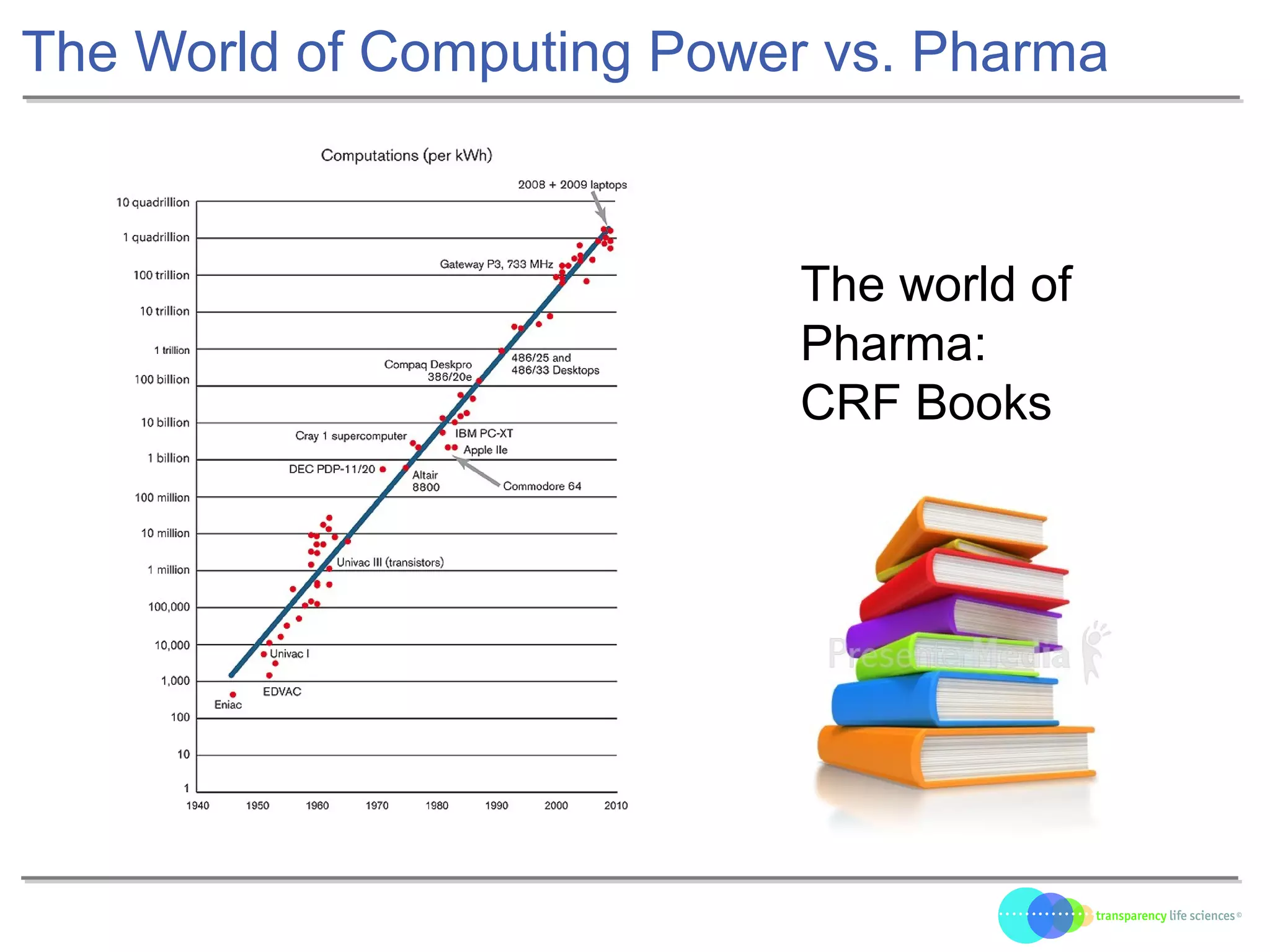
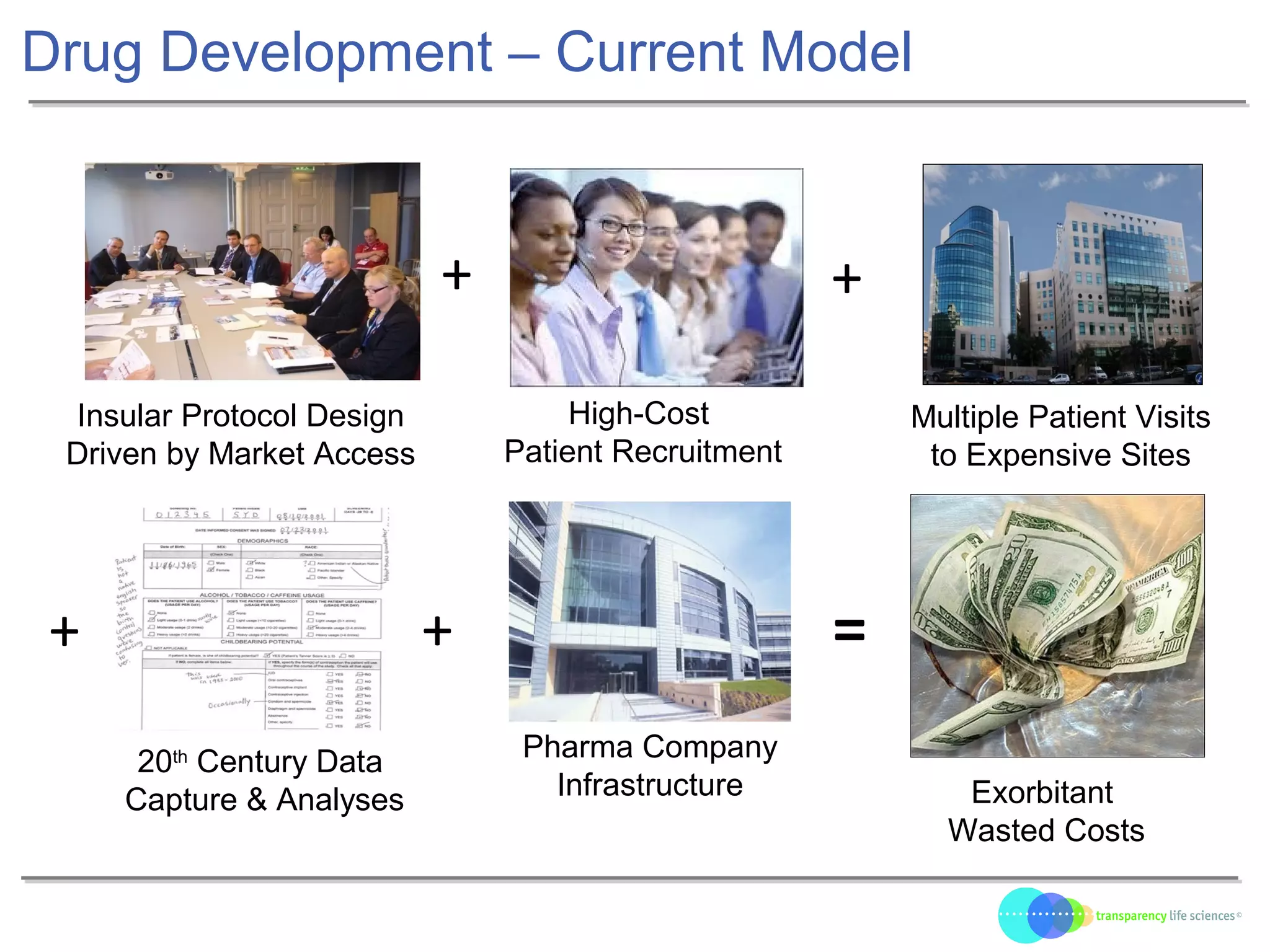
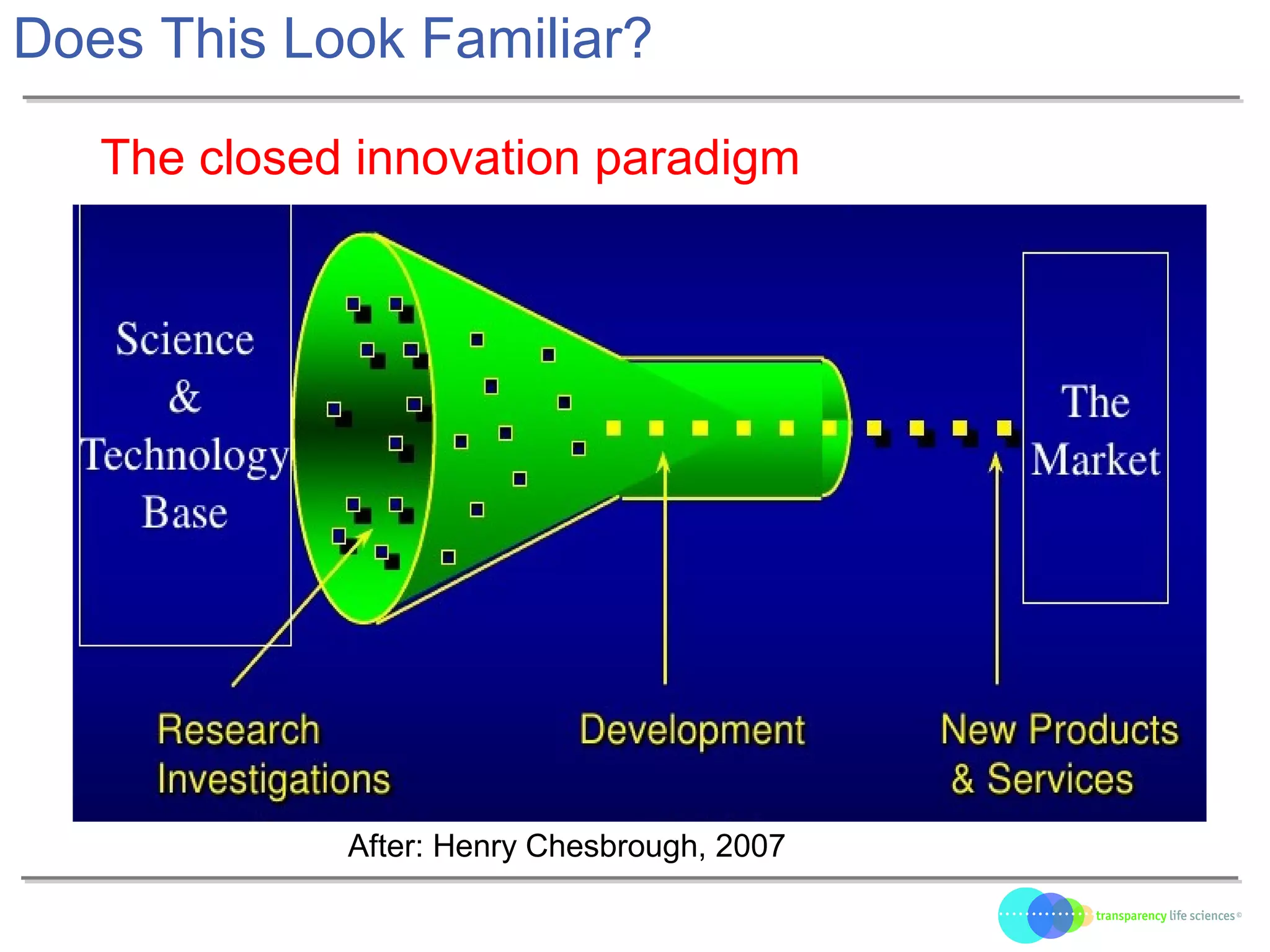
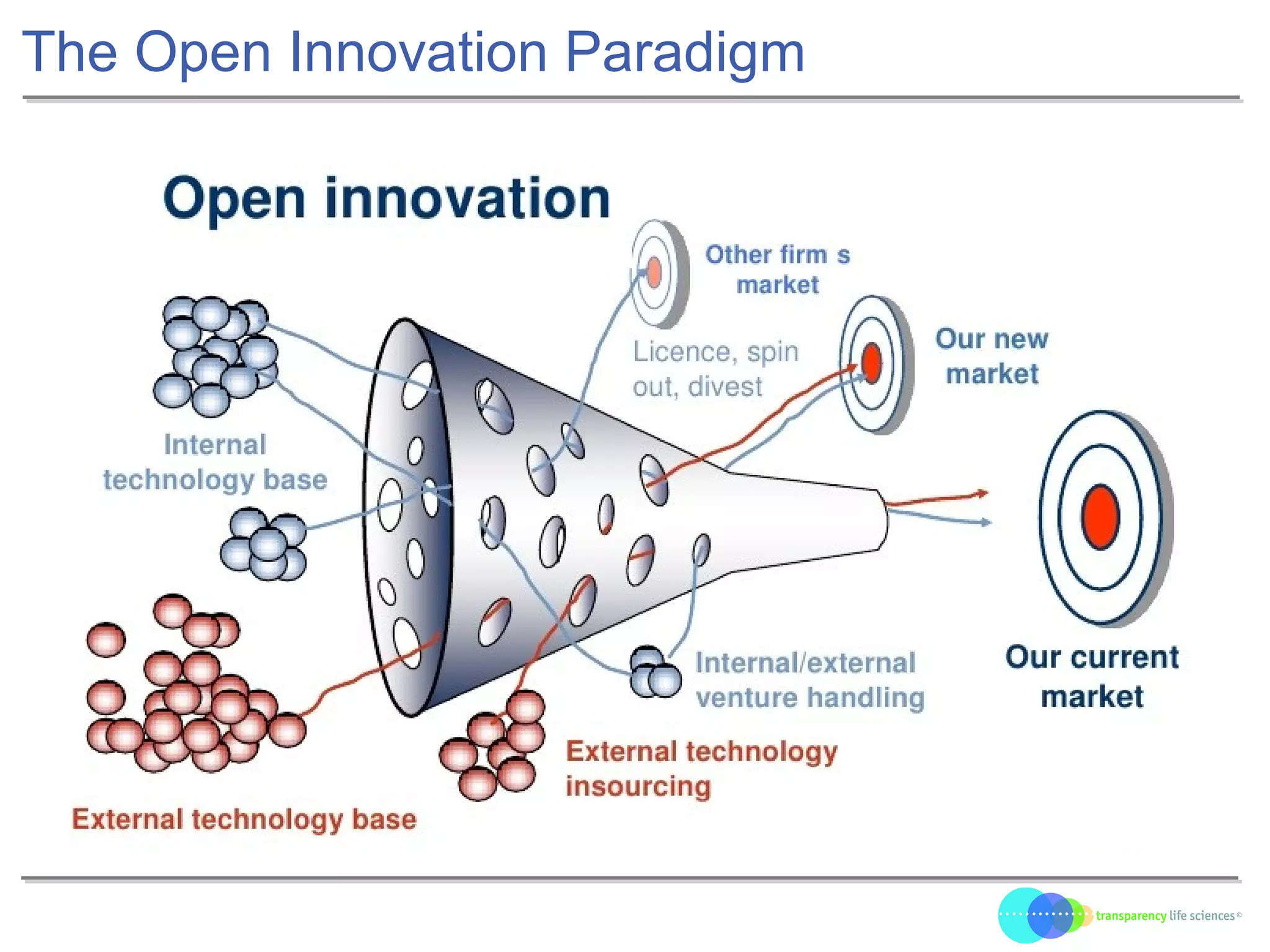
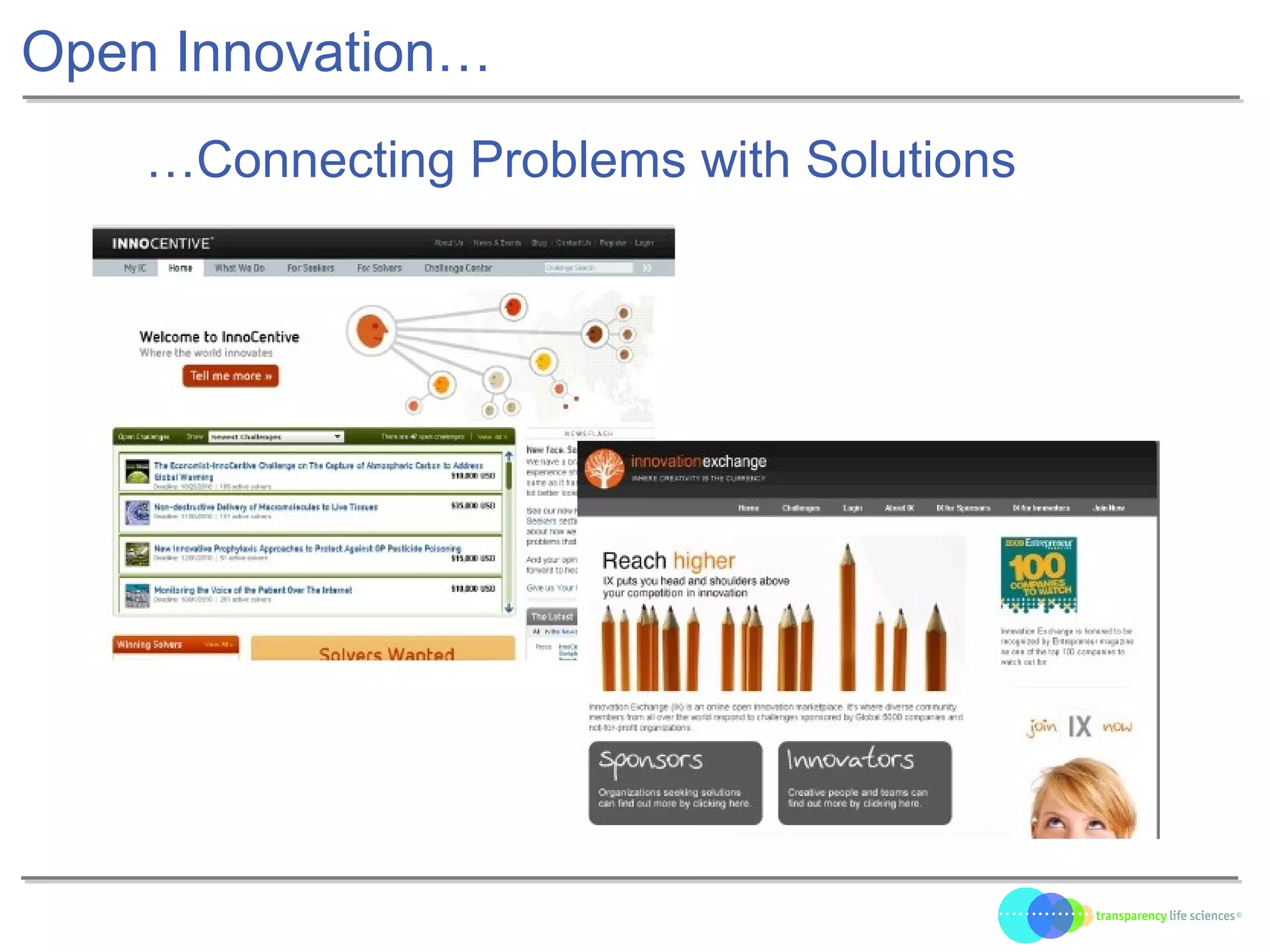
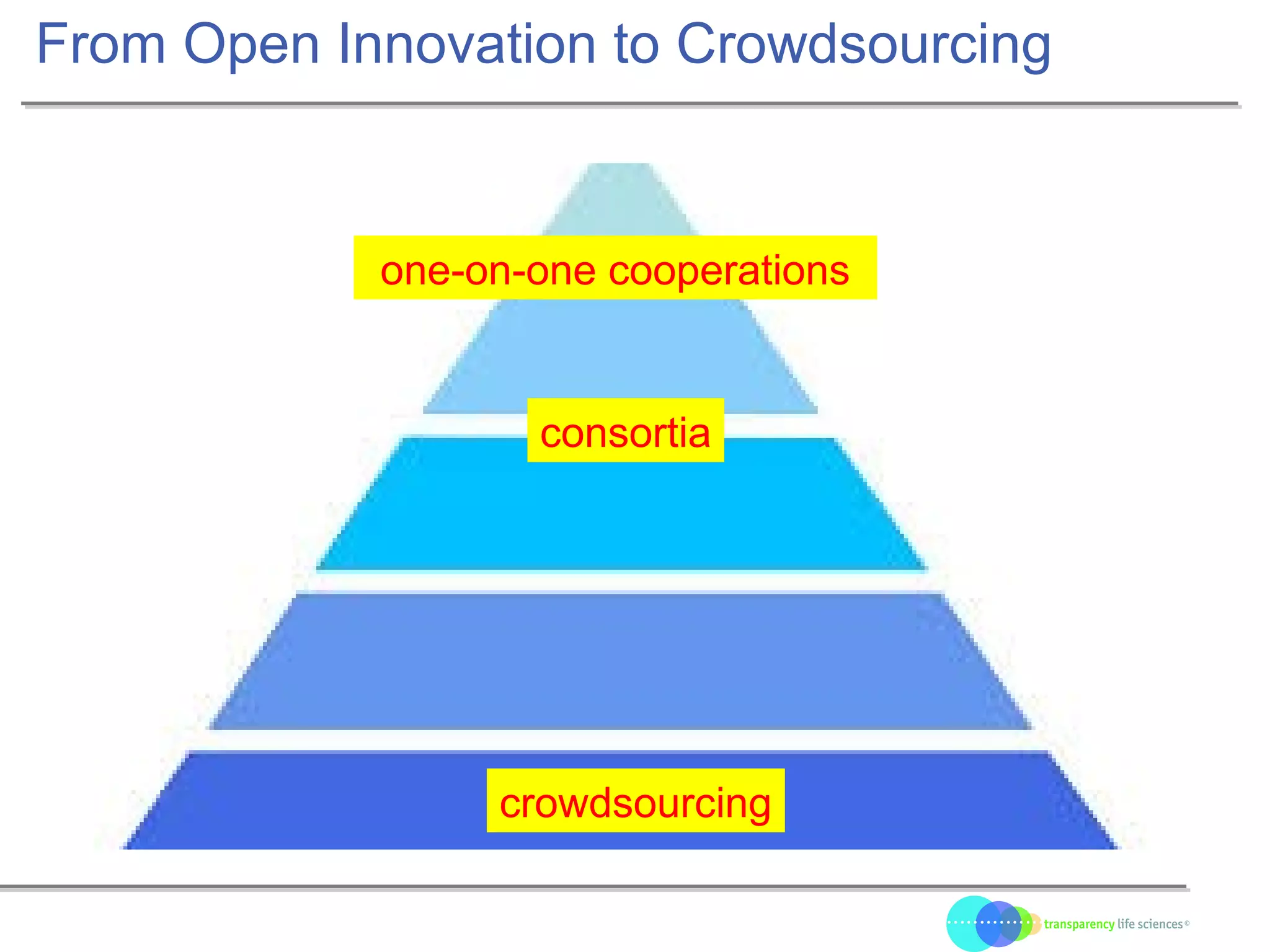
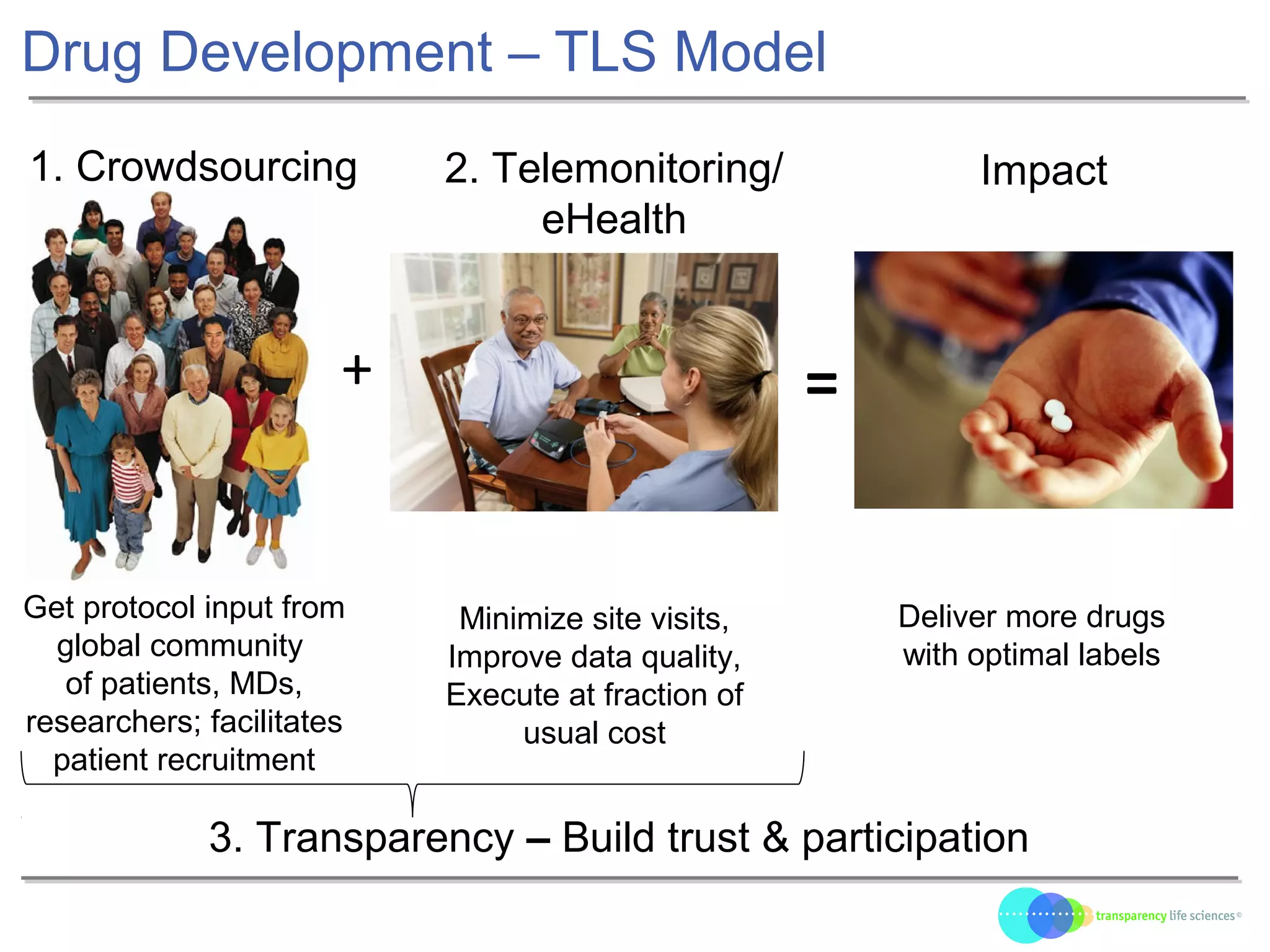
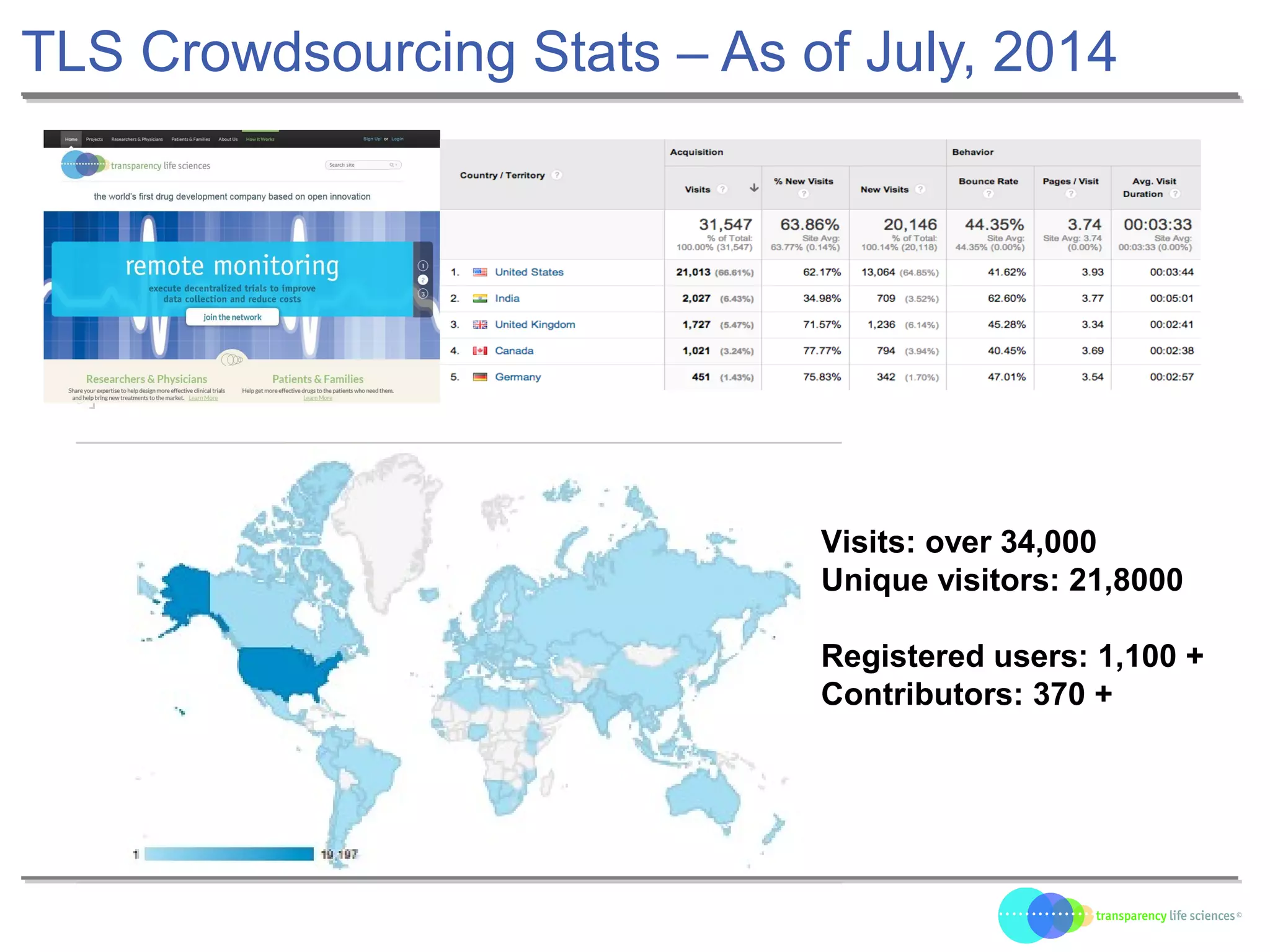
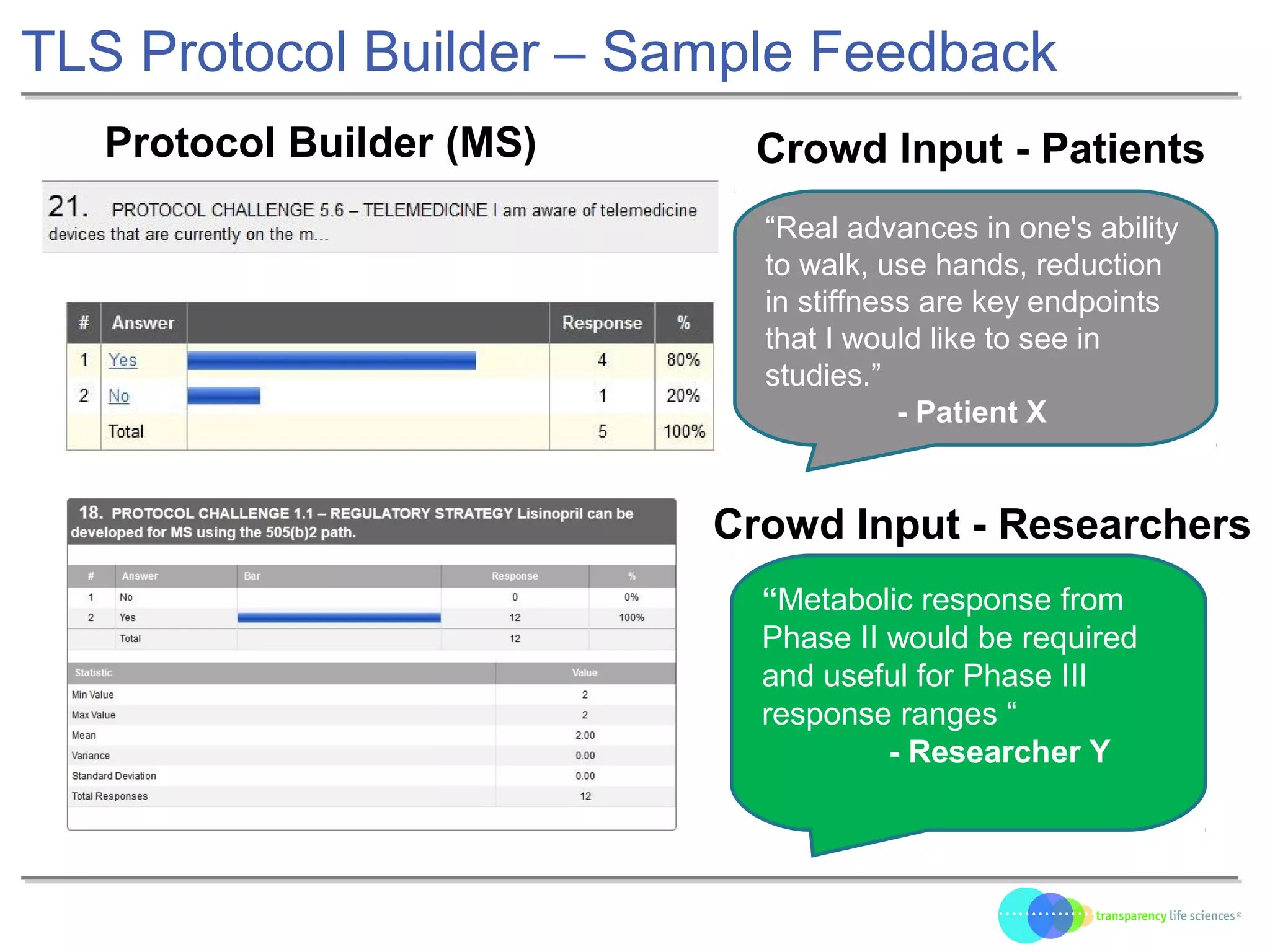
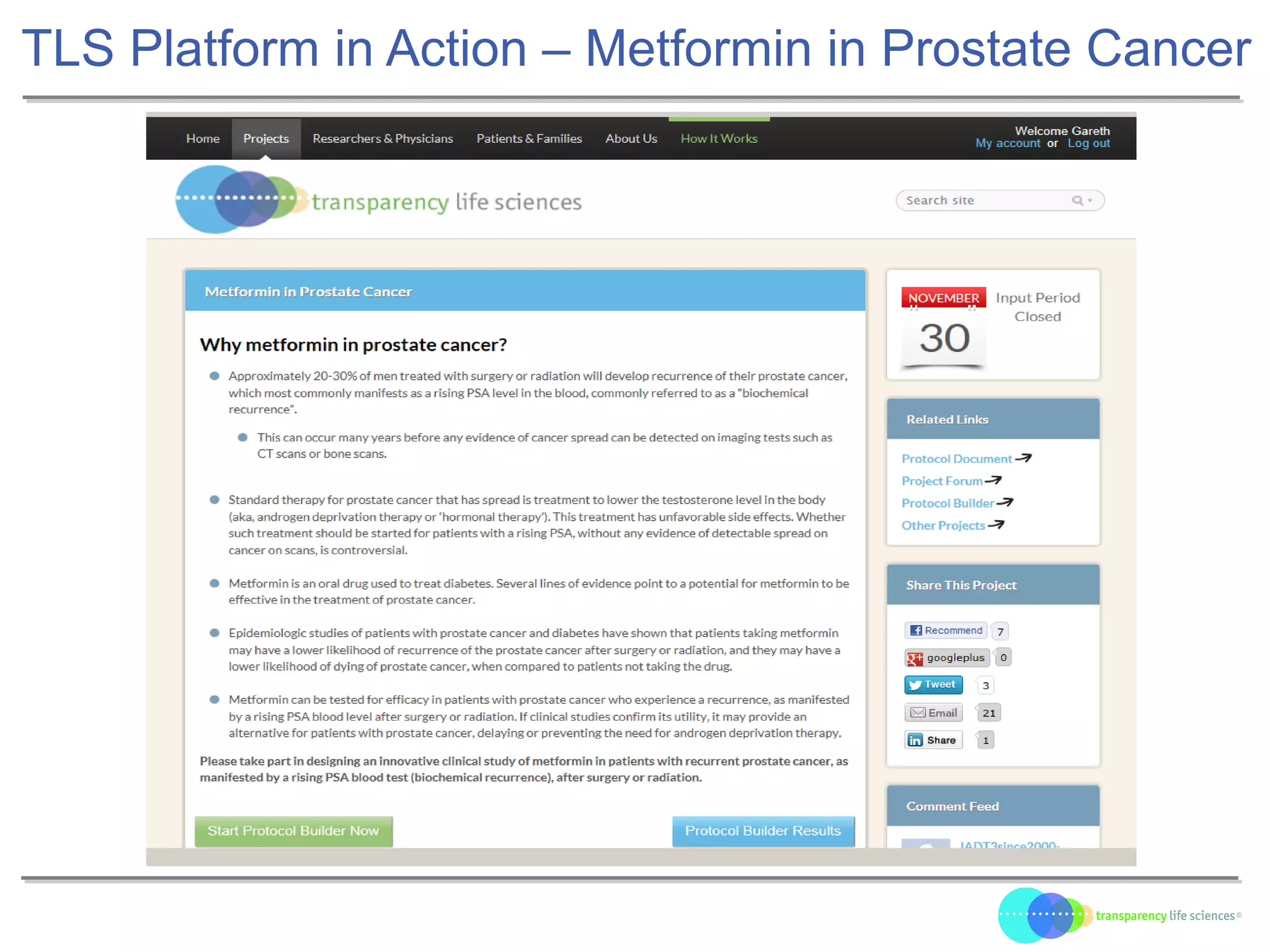
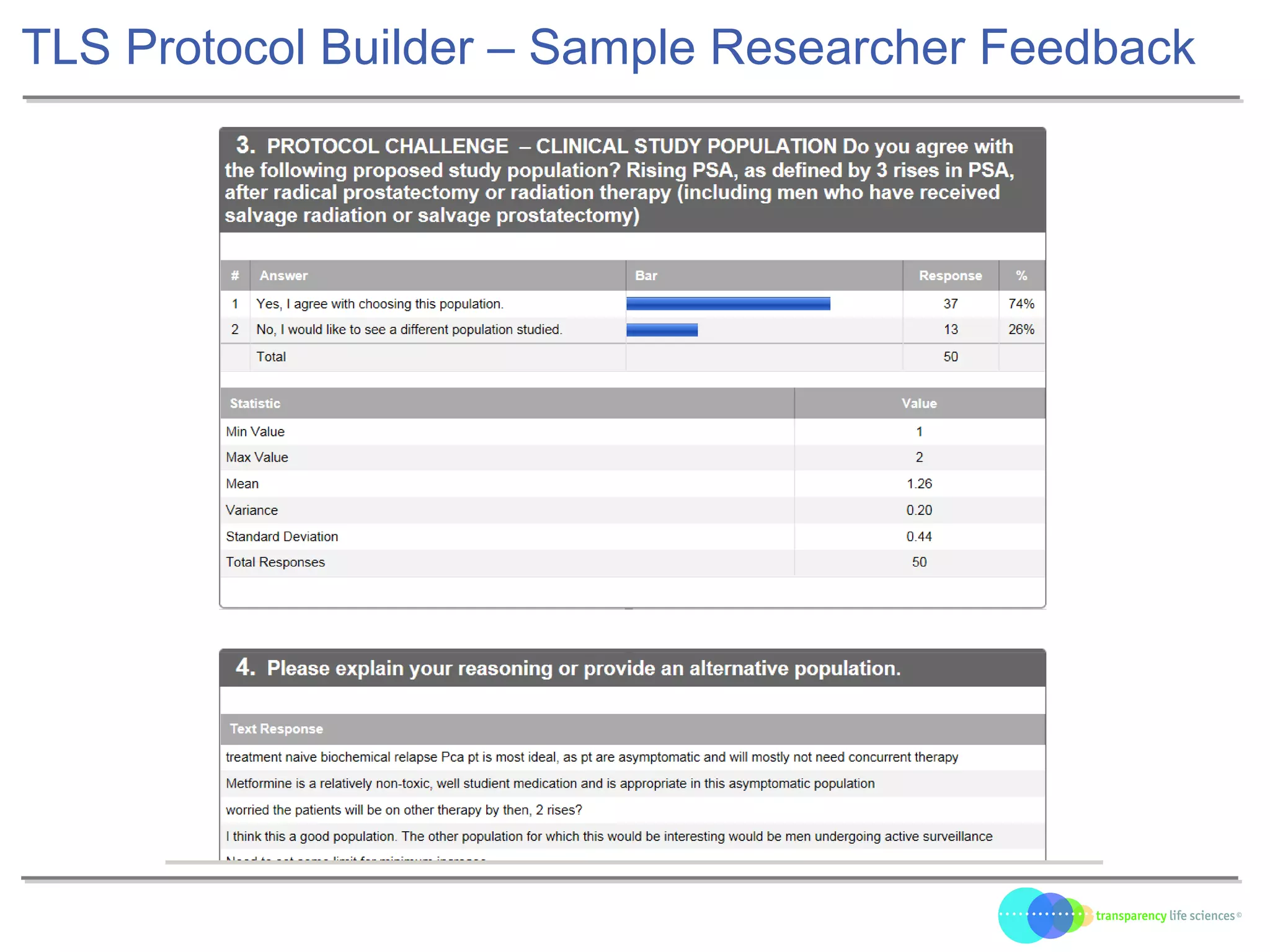
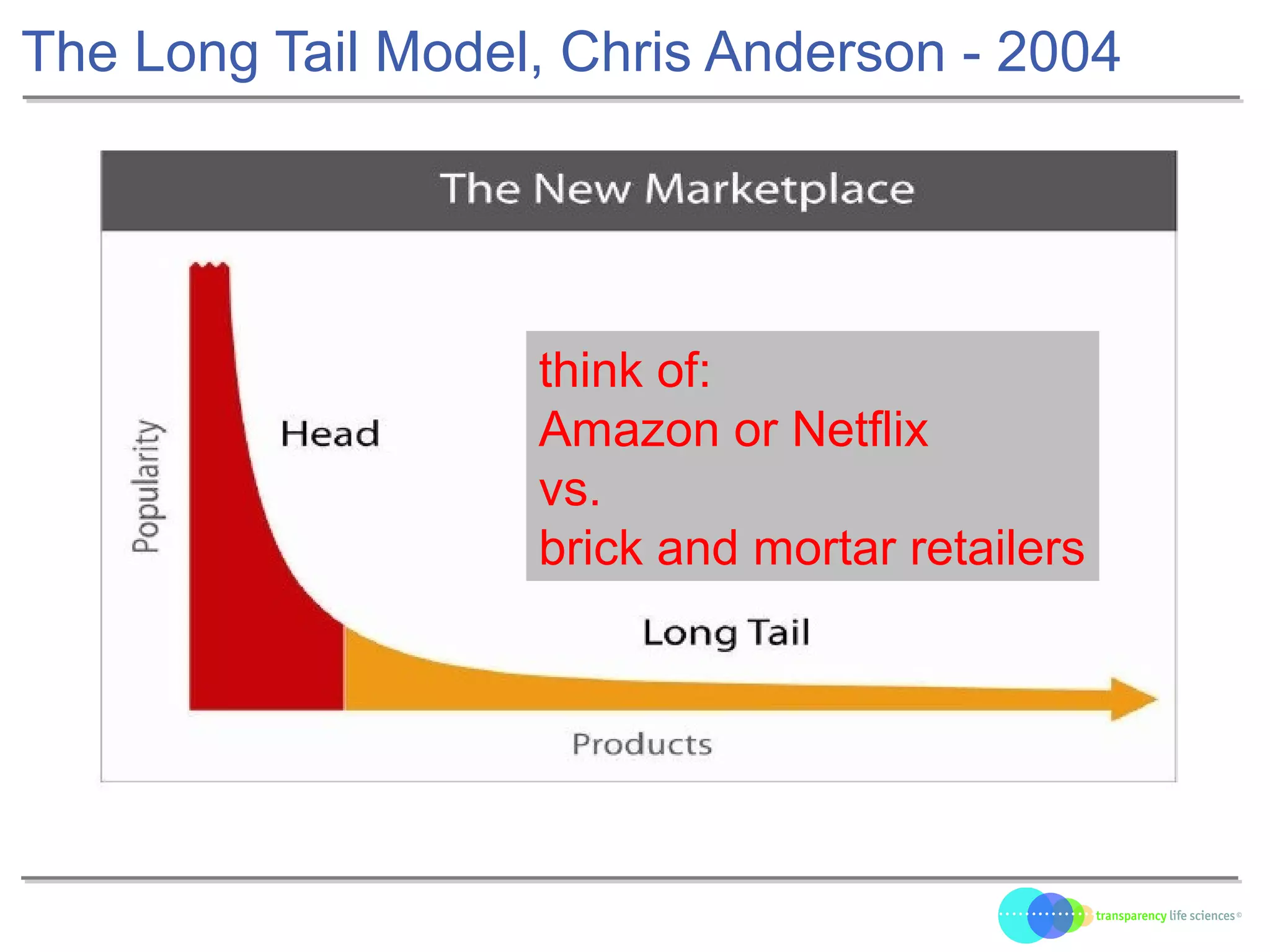
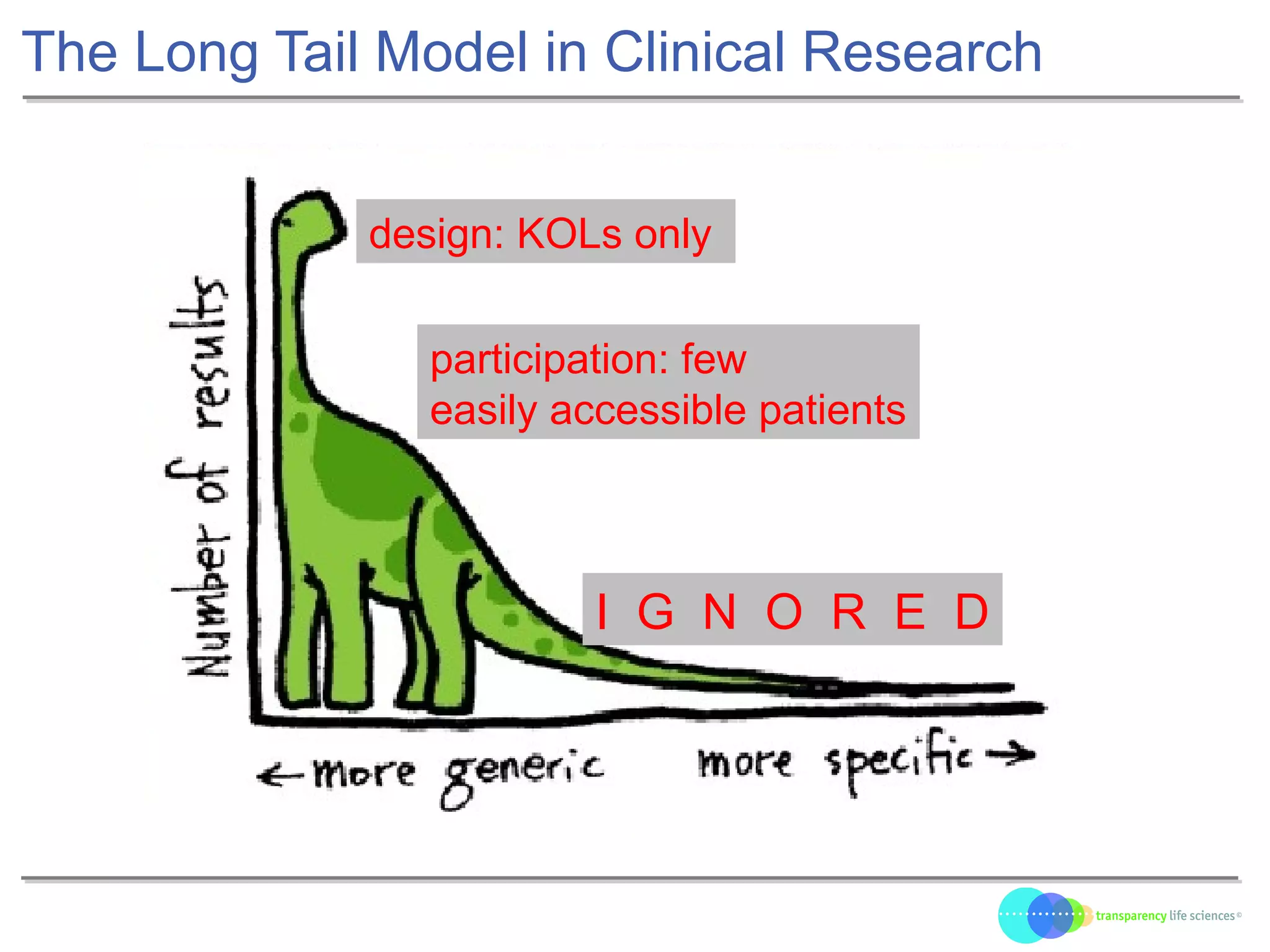
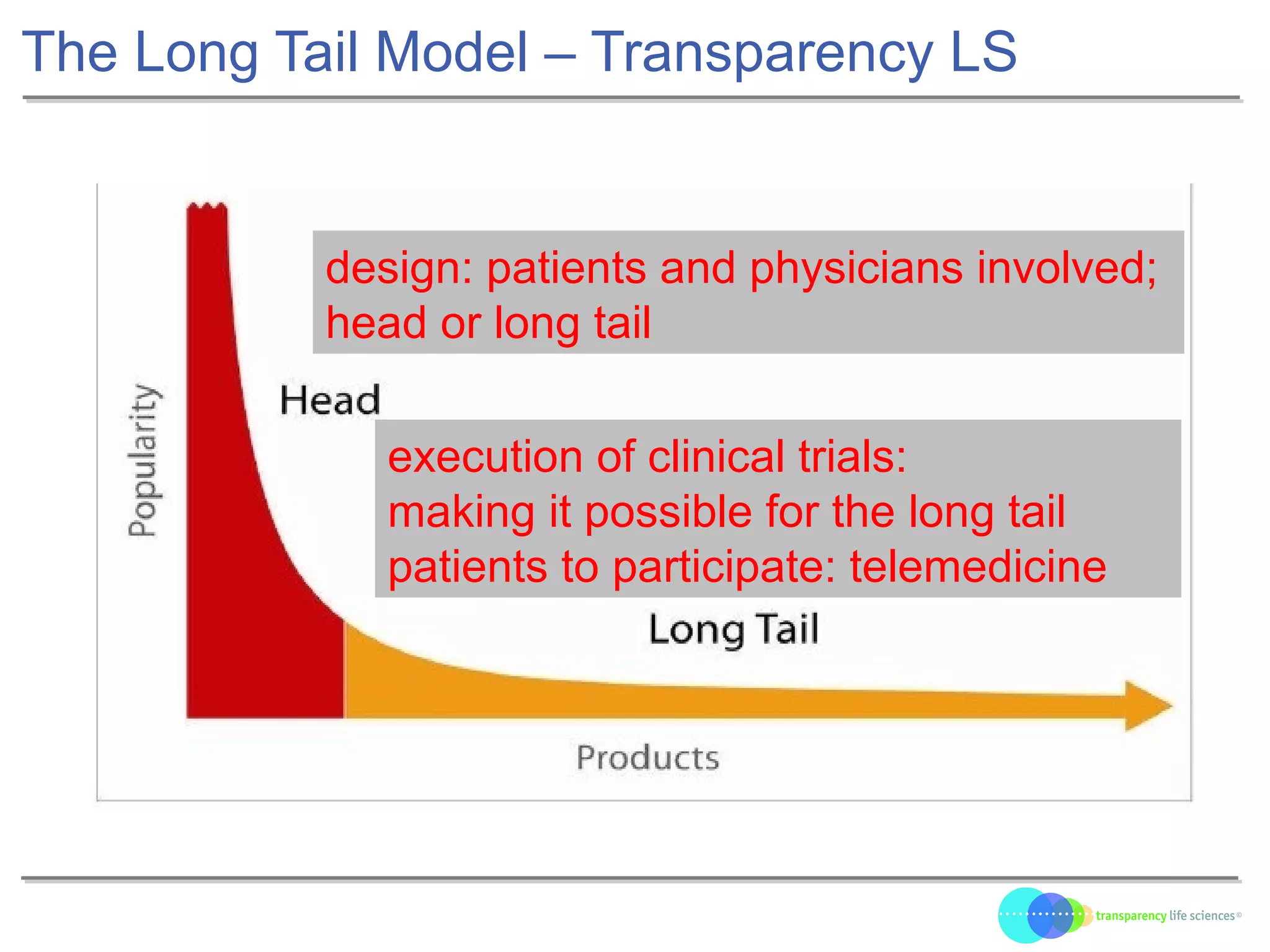
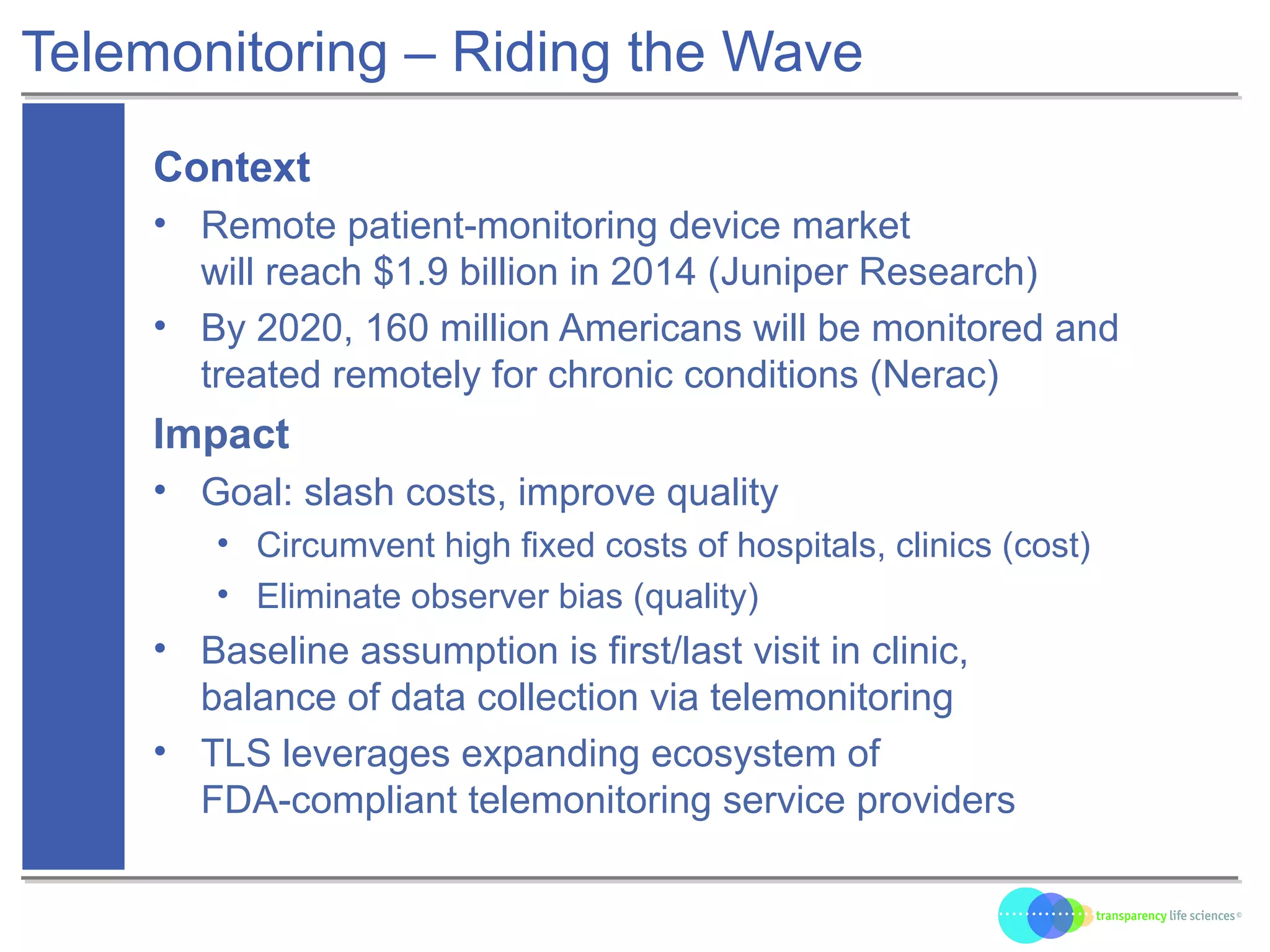
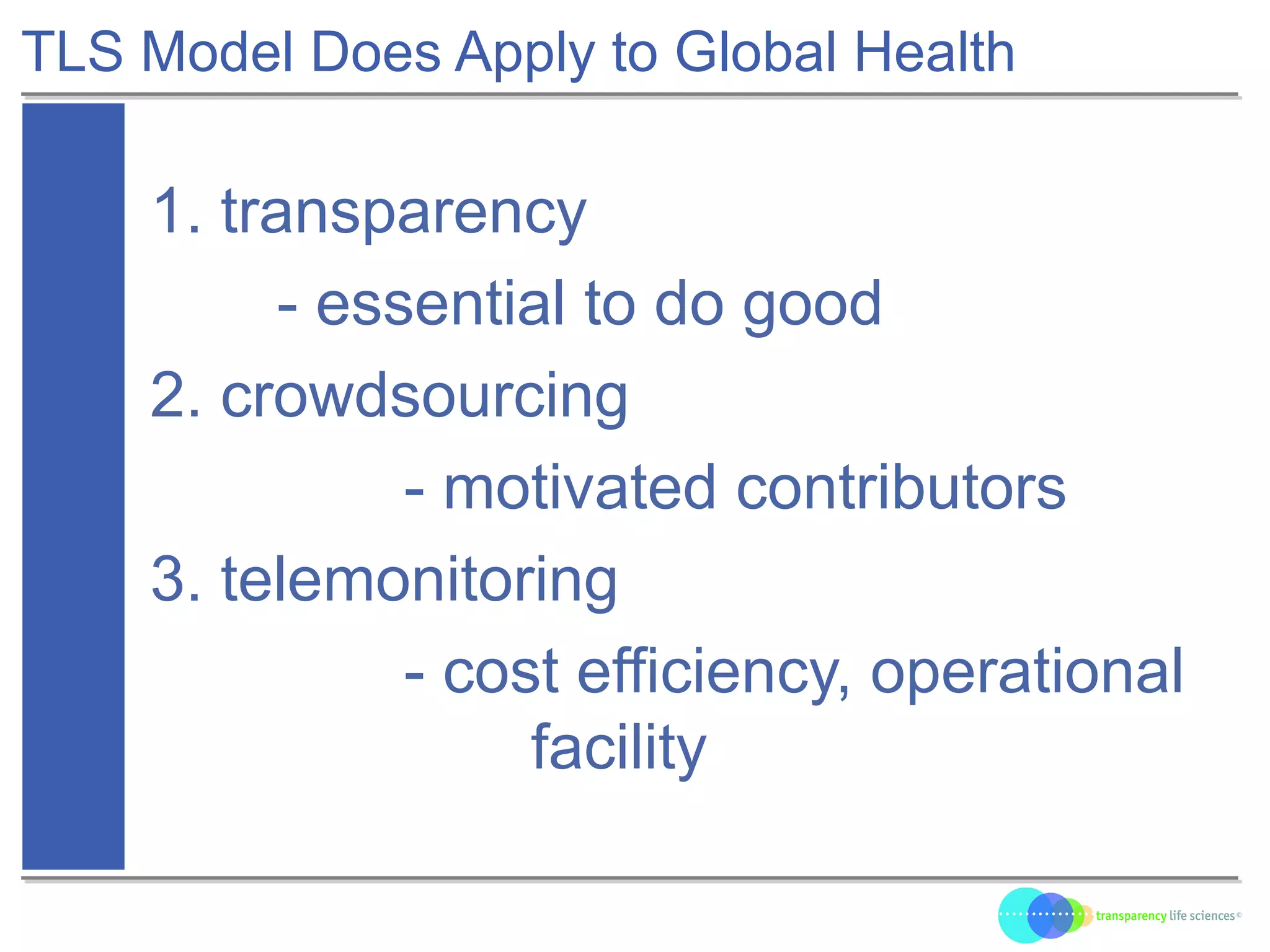
The document discusses the evolution of drug development through open innovation, emphasizing the need for transparency, crowdsourcing, and telemonitoring to enhance clinical trials. It highlights the inefficiencies of the current closed model and introduces the TLS model as a more cost-effective and participatory approach to drug development. The integration of modern technology aims to improve patient recruitment and data quality while reducing costs in the pharmaceutical industry.