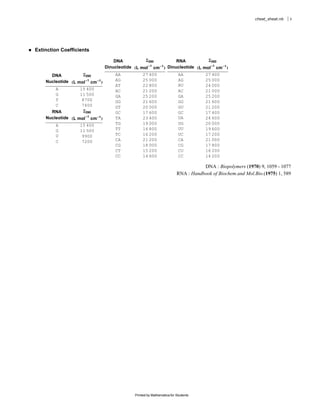
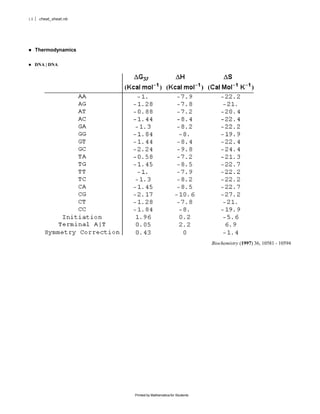
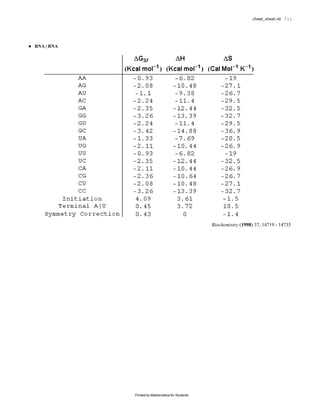
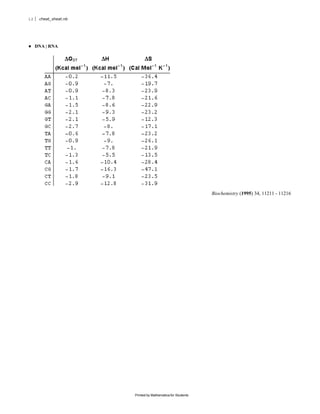
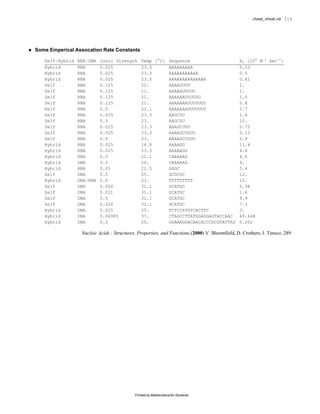
The document provides equations and definitions relating to mass, ionic strength, extinction coefficients, thermodynamics, kinetics, and empirical rate constants for nucleic acid hybridization and structure. Specifically, it defines equations for mass, ionic strength, linear and circular dichroism extinction coefficients, Gibbs free energy, equilibrium distributions for single and multiple equilibria, first and second order reaction kinetics, melting temperatures, and provides sample empirical association rate constants for RNA and DNA self-hybridization.