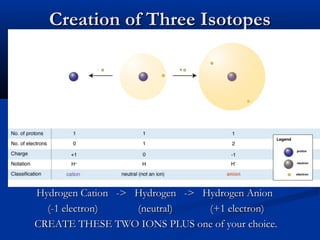
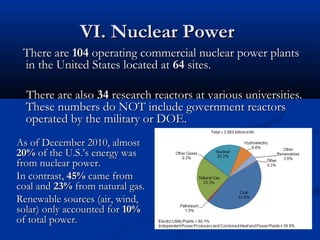
Three types of particles are defined: alpha particles, beta particles, and gamma rays. Particle accelerators like the Tevatron and LHC are used to study particle interactions by accelerating them to high speeds and observing collision results. The goals are to better understand fundamental forces and the structure of space and time.