This document summarizes several key points regarding the management of NSTEMI and the use of ticagrelor vs clopidogrel:
1) ACS patients in the Gulf region are younger than in developed countries and often present late to the hospital. Mortality among NSTEMI patients is higher in those with multi-vessel disease.
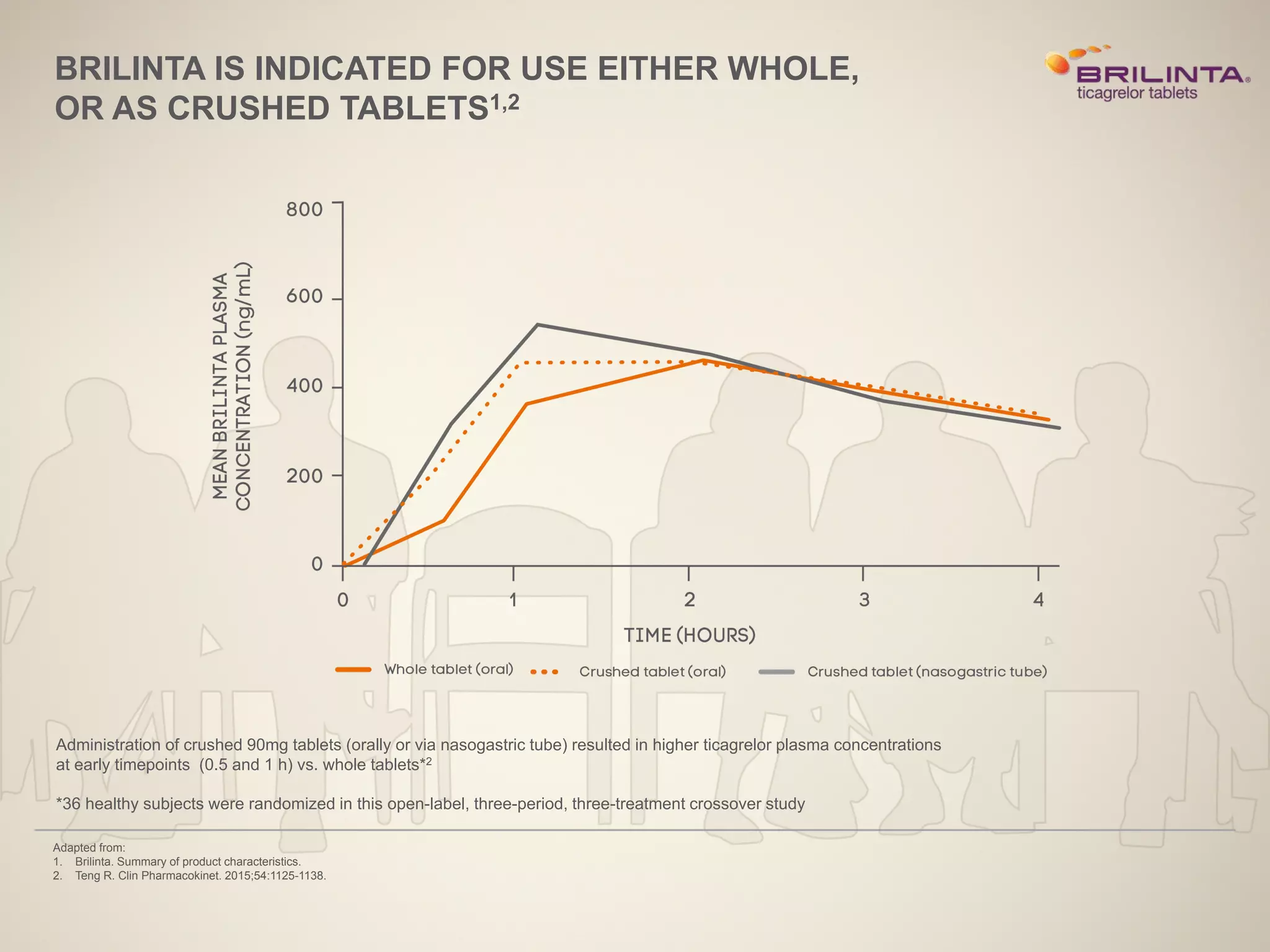
2) The PLATO trial demonstrated that ticagrelor 90mg reduced cardiovascular mortality by 21% compared to clopidogrel and showed efficacy in NSTE-ACS patients with no increase in overall major bleeding.
3) The PRACTICAL study of over 45,000 real-world ACS patients found benefits of ticagrelor 90mg


















![PRACTICAL
EVALUATION OF OUTCOMES IN A LARGE POPULATION OF REAL-WORLD ACS
PATIENTS TREATED WITH BRILINTA 90MG OR CLOPIDOGREL19
Adapted from:
19. Sahlén A, et al. Eur Heart J 2016; pii: ehw284 [Epub ahead of print].
Due to BRILINTA 90mg 12 months license, only 12 months sensitivity analysis of the primary endpoints can be shared during this presentation
for efficacy analysis.
Data from PRACTICAL, an observational study of 45,073 patients prospectively enrolled into the SWEDEHEART registry, patients who were discharged on either BRILINTA 90mg or
clopidogrel were evaluated for the composite primary endpoints of death, MI or stroke.19](https://image.slidesharecdn.com/nstemimvdpromotionalslidesupdate1-230609155504-0fd63398/75/NSTEMI-MVD-Promotional-Slides-Update-1-pptx-19-2048.jpg)
![PRACTICAL
STUDY DESIGN
Adapted from:
19. Sahlén A, et al. Eur Heart J 2016; pii: ehw284 [Epub ahead of print].
Data from PRACTICAL, an observational study of 45,073 patients prospectively enrolled into the SWEDEHEART registry, patients who were
discharged on either BRILINTA 90mg or clopidogrel were evaluated for the composite primary endpoints of death, MI or stroke.](https://image.slidesharecdn.com/nstemimvdpromotionalslidesupdate1-230609155504-0fd63398/75/NSTEMI-MVD-Promotional-Slides-Update-1-pptx-20-2048.jpg)
![PRACTICAL
BRILINTA WAS RAPIDLY ADOPTED IN SWEDEN FOLLOWING ITS LAUNCH AND
INCLUSION IN ESC GUIDELINES19
Adapted from:
19. Sahlén A, et al. Eur Heart J 2016; pii: ehw284 [Epub ahead of print].
WITHIN 9 MONTHS OF LAUNCH BRILINTA HAS ACHIEVED ALMOST 50% SHARE IN DAPT MARKET19
Data from PRACTICAL, an observational study of 45,073 patients prospectively enrolled into the SWEDEHEART registry, patients who were
discharged on either BRILINTA 90mg or clopidogrel were evaluated for the composite primary endpoints of death, MI or stroke.](https://image.slidesharecdn.com/nstemimvdpromotionalslidesupdate1-230609155504-0fd63398/75/NSTEMI-MVD-Promotional-Slides-Update-1-pptx-21-2048.jpg)
![PRACTICAL
BENEFITS OF BRILINTA 90MG IN PRACTICAL VS. CLOPIDOGREL SHOWED
CONSISTENCY WITH PLATO SECONDARY ENDPOINT IN A REAL-WORLD
SETTING AT 12 MONTHS12,18
Adapted from:
12. Wallentin L, et al. N Engl J Med 2009; 361: 1045-1057
18. Sahlén A, et al. Eur Heart J 2016; pii: ehw284 [Epub ahead of print].
BRILINTA 90MG WAS ASSOCIATED WITH A 0.85% HAZARD RATIO IN THE COMPOSITE OF ALL-CAUSE
DEATH, MI AND STROKE VS. CLOPIDOGREL AT 12 MONTHS12
*Graph adapted with 12-month sensitivity analysis data performed by PRACTICAL study team.
Data from PRACTICAL, an observational study of 45,073 patients prospectively enrolled into the SWEDEHEART registry. BRILINTA 90mg was associated
with a lower risk of all-cause death, MI or stroke vs. clopidogrel (crude rates 11.7% vs. 22.3%, respectively, with a multivariate risk adjusted HR 0.85, 95%
CI 0.78-0.93). Final values are adjusted for hospital and calendar time, age, sex, discharge medication, in-hospital course, presentation characteristics and
pre-existing comorbidities. As an observational study of real-world evidence, the data from this study are subject to potential confounding bias usually
associated with observational research. Data are for treatment at discharge and planned treatment duration only.18](https://image.slidesharecdn.com/nstemimvdpromotionalslidesupdate1-230609155504-0fd63398/75/NSTEMI-MVD-Promotional-Slides-Update-1-pptx-22-2048.jpg)