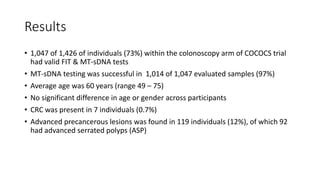
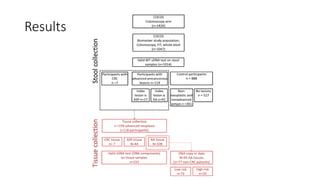
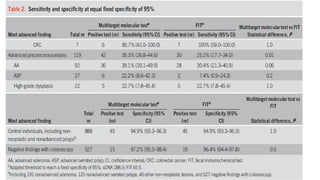
This study compared the performance of a multitarget stool DNA test (MT-sDNA) versus a fecal immunochemical test (FIT) for colorectal cancer screening in an average-risk population. MT-sDNA had higher sensitivity than FIT for detecting colorectal cancer and advanced precancerous lesions, but lower specificity. Using a fixed specificity of 95%, MT-sDNA sensitivity was higher than FIT for detecting cancers and advanced lesions. While neither test was more sensitive for detecting specific lesion characteristics, MT-sDNA and FIT may provide complementary benefits when used together for colorectal cancer screening.