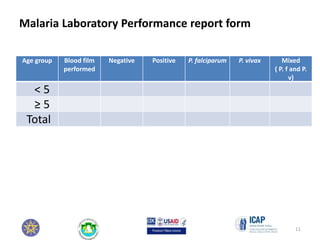
This module focuses on the recording and reporting of blood film results, outlining essential elements such as accuracy, completeness, and data entry into laboratory registers. Participants will learn to correctly report microscopy results and maintain up-to-date records for effective resource planning and epidemiological data compilation. Key documentation includes the laboratory request form, microscopy report, and malaria performance report, all of which aid in tracking and analyzing malaria cases.