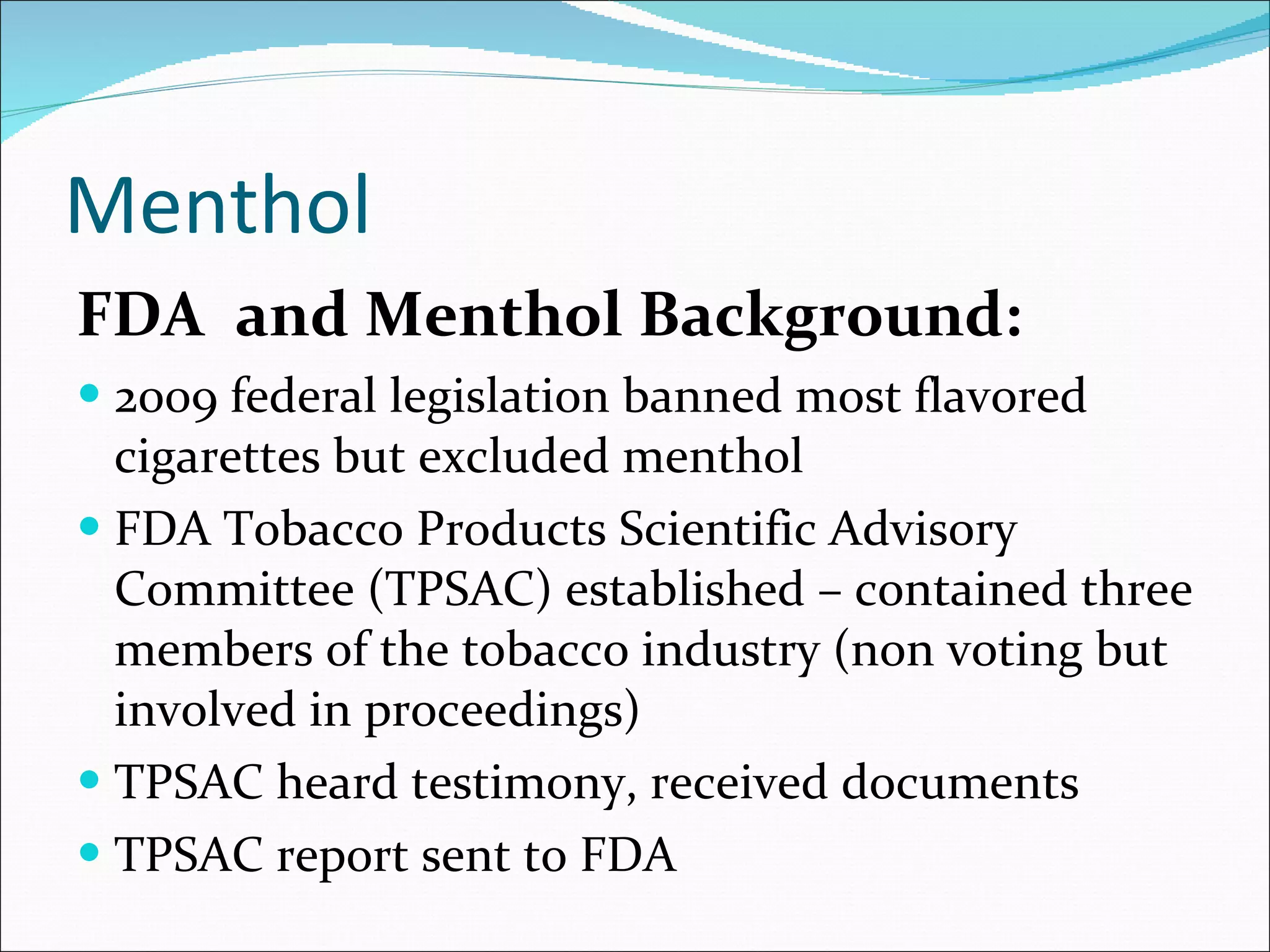
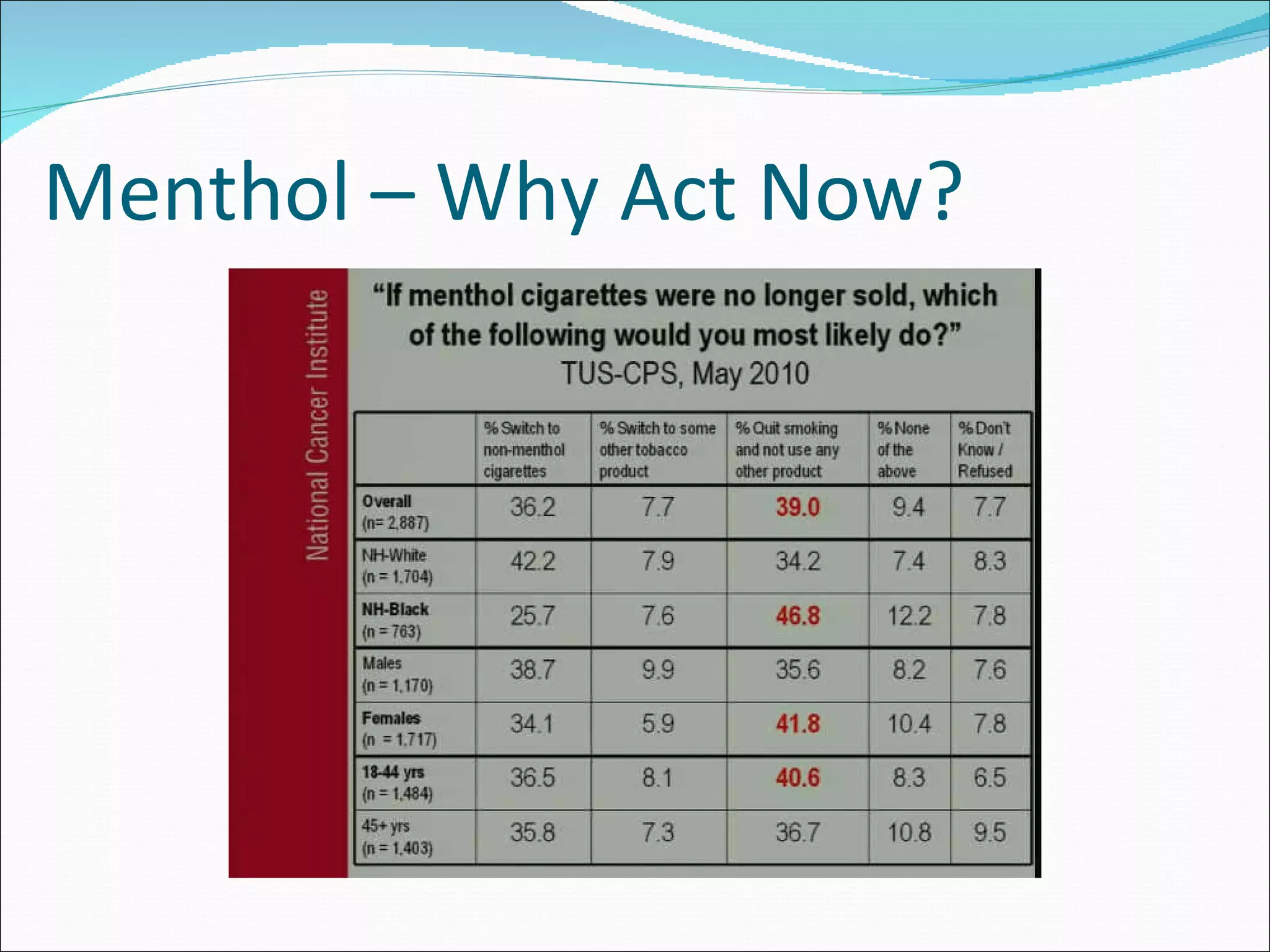
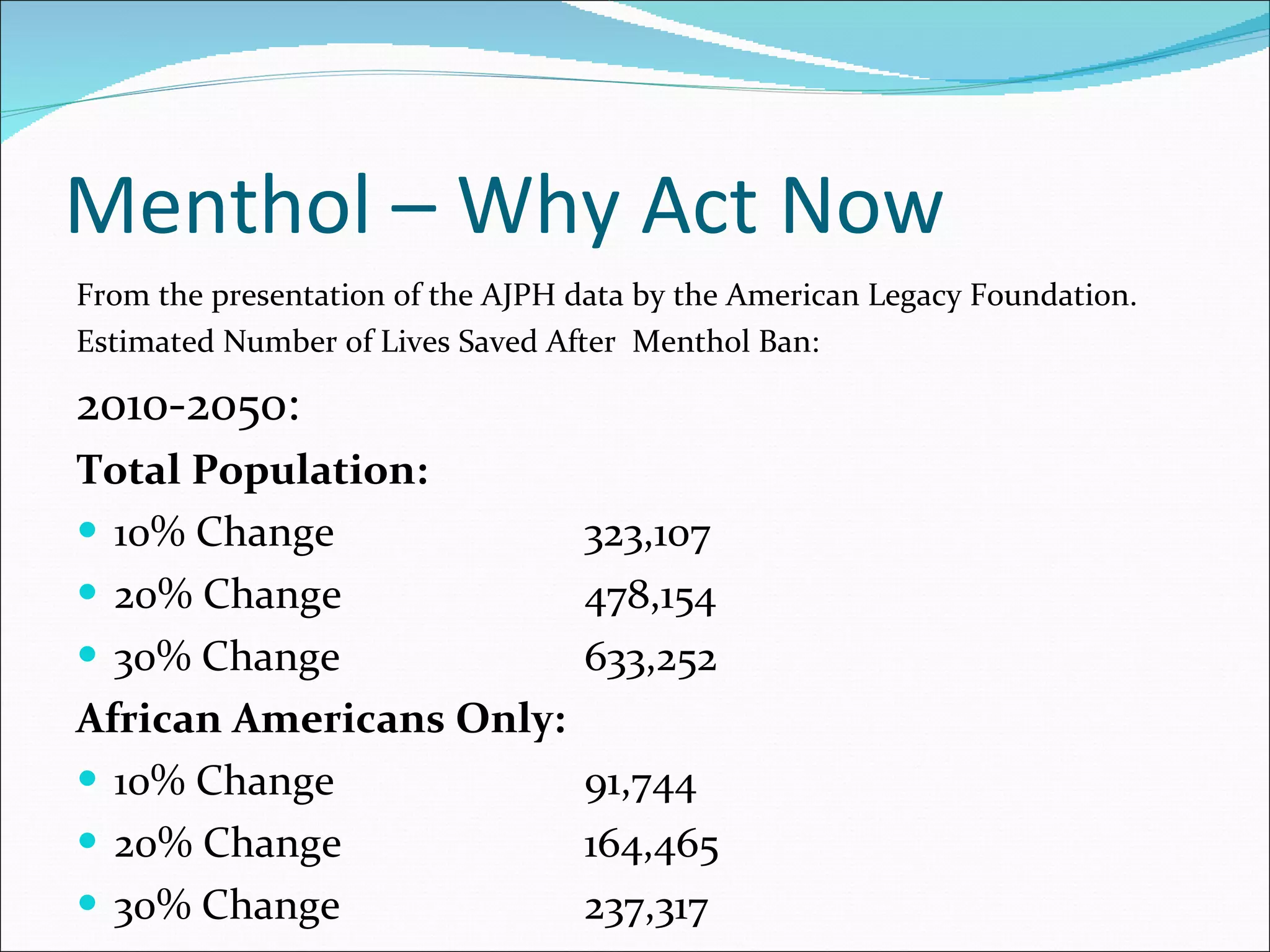
The document discusses menthol cigarettes and calls for the FDA to remove menthol flavoring from all tobacco products. It provides background on menthol cigarette use, the tobacco industry's targeting of certain groups, and strategies being used to oppose a menthol ban. Recent news and studies suggest removing menthol could significantly reduce smoking rates and tobacco-related deaths over time, especially among African Americans. The next steps mentioned are the upcoming FDA report and TPSAC meeting to further consider the issues.

![Menthol Monday Purpose Purpose: Assemble individuals and organizations interested in having the FDA take action and remove menthol flavoring from all tobacco products. Menthol Mondays calls focus: Remain current on news and issues Develop or support strategies to engage/educate/mobilize the public/communities, decision makers, businesses/organizations, media Communicate and collaborate on local, state, and federal strategies To be part of the calls, get updates, e-mail Bob Doyle at [email_address] Menthol Monday call/webinar is the 3 rd Monday of each month](https://image.slidesharecdn.com/menthol-monday-webinar-summaryppt-1309874100-phpapp02-110705085648-phpapp02/75/Menthol-Monday-Webinar-Summary-Ppt-2-2048.jpg)





















![Next Steps FDA June Report Due TPSAC Meeting July 21 to consider report amendments How can you/your organization help? Conduct community presentations, distribute material to community/state organizations Have your organization/its web site support menthol’s removal Have a presence at large community events Pass the word/invite others to the Next Menthol Monday call July 18 at noon – contact Bob Doyle – [email_address]](https://image.slidesharecdn.com/menthol-monday-webinar-summaryppt-1309874100-phpapp02-110705085648-phpapp02/75/Menthol-Monday-Webinar-Summary-Ppt-28-2048.jpg)