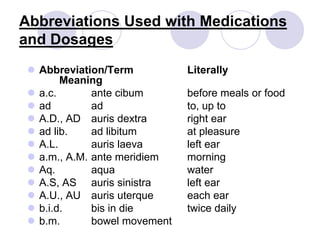
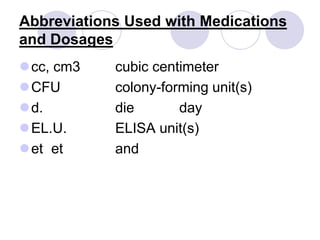
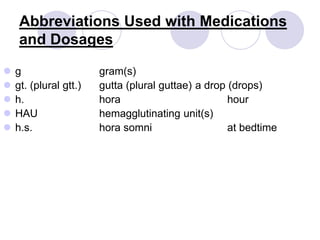
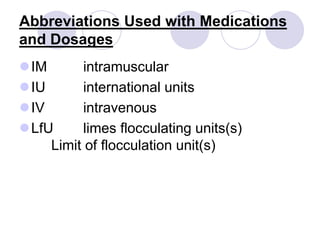
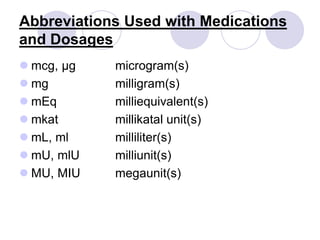
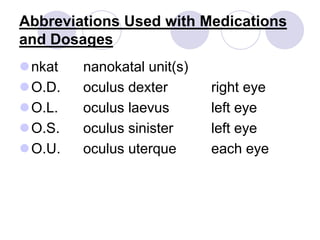
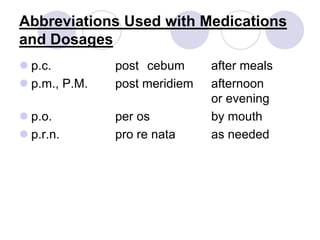
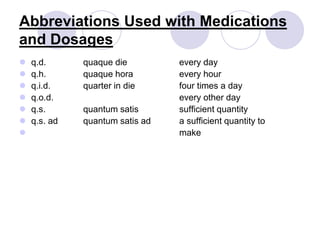
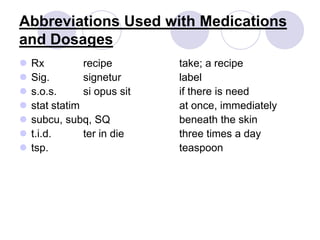
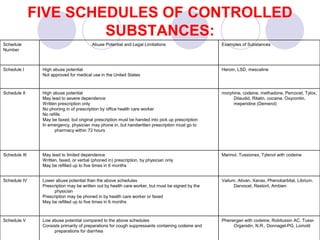
This document provides an overview of a pharmacology course covering drug definitions, classifications, names, and information sources. It discusses the sources and effects of drugs in the body, safe medication preparations, and abbreviations used in prescriptions. Key topics include the five schedules of controlled substances based on abuse potential and legal restrictions, as well as definitions of terms related to drug actions, regulations, and laws governing drugs.