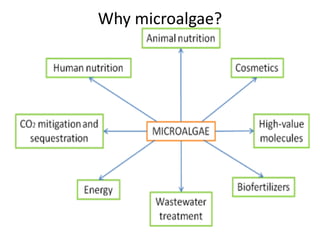
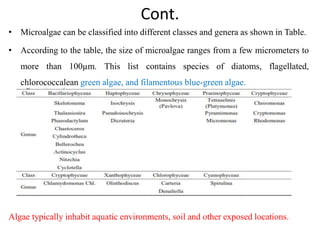
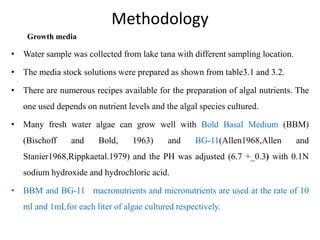
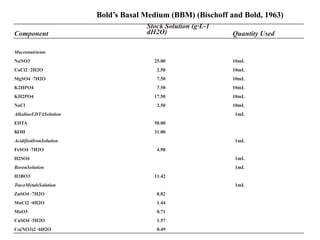
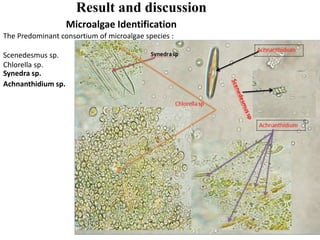
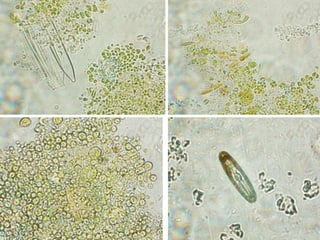
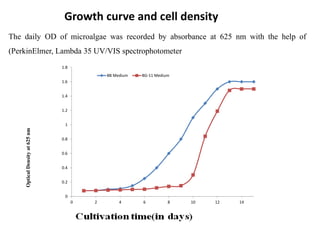
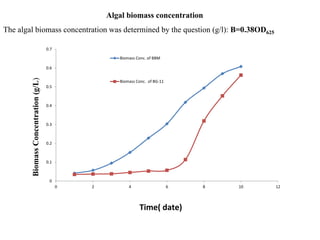
This thesis investigates the potential of microalgae species to biodegrade reactive blue dye. The study aims to identify dominant microalgae species in Bahir Dar, Ethiopia and examine their ability to degrade dye under different conditions. The methodology involves growing microalgae in culture media, identifying the predominant species, and conducting experiments to determine the effect of culturing medium, substrate concentration, and contact time on dye degradation. Preliminary results found the dominant microalgae species to be Scenedesmus sp., Chlorella sp., Synedra sp., and Achnanthidium sp. Growth curves showed higher biomass concentration when cultured in BBM medium compared to BG-11 medium. Further analysis of dye