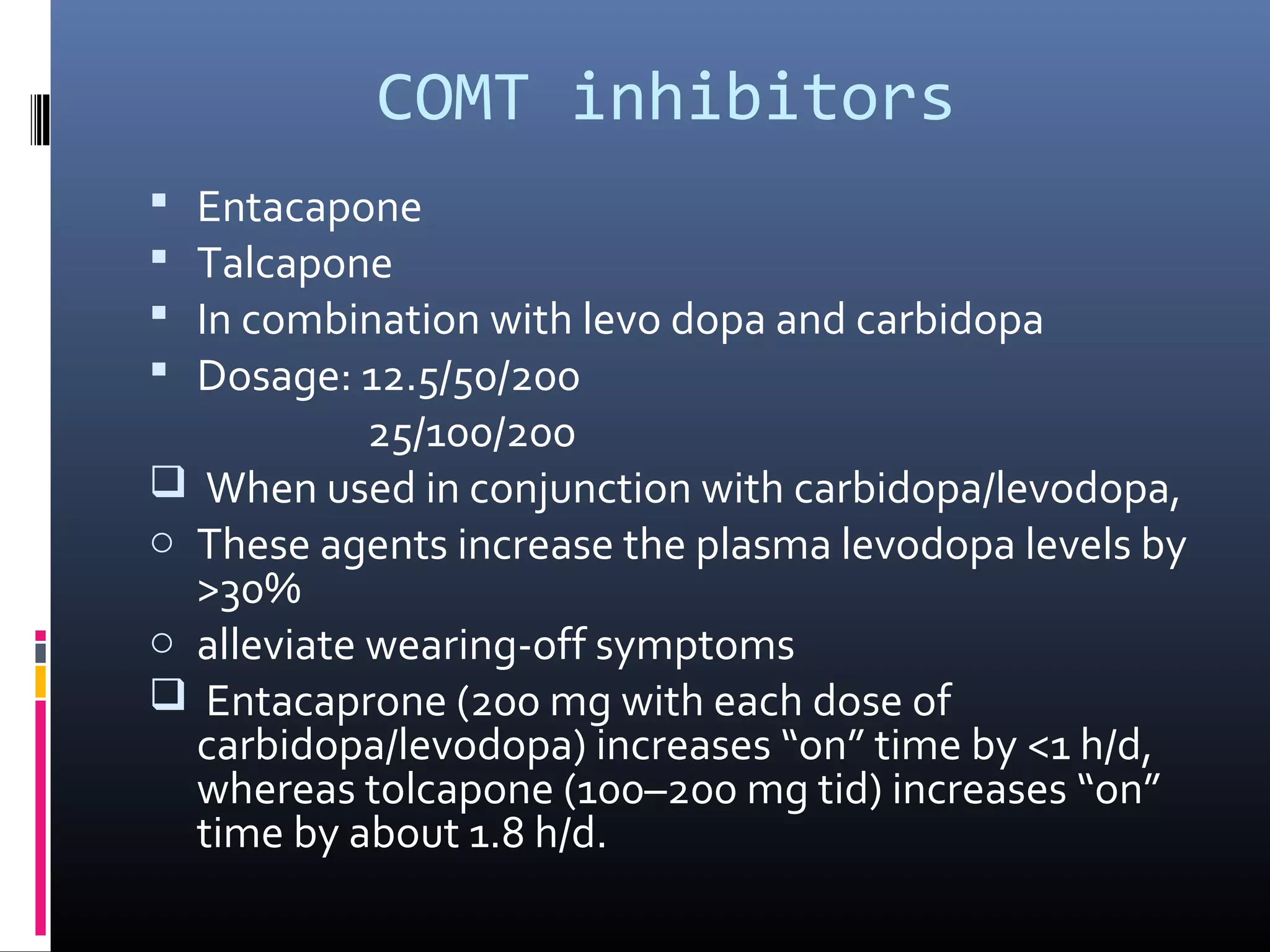
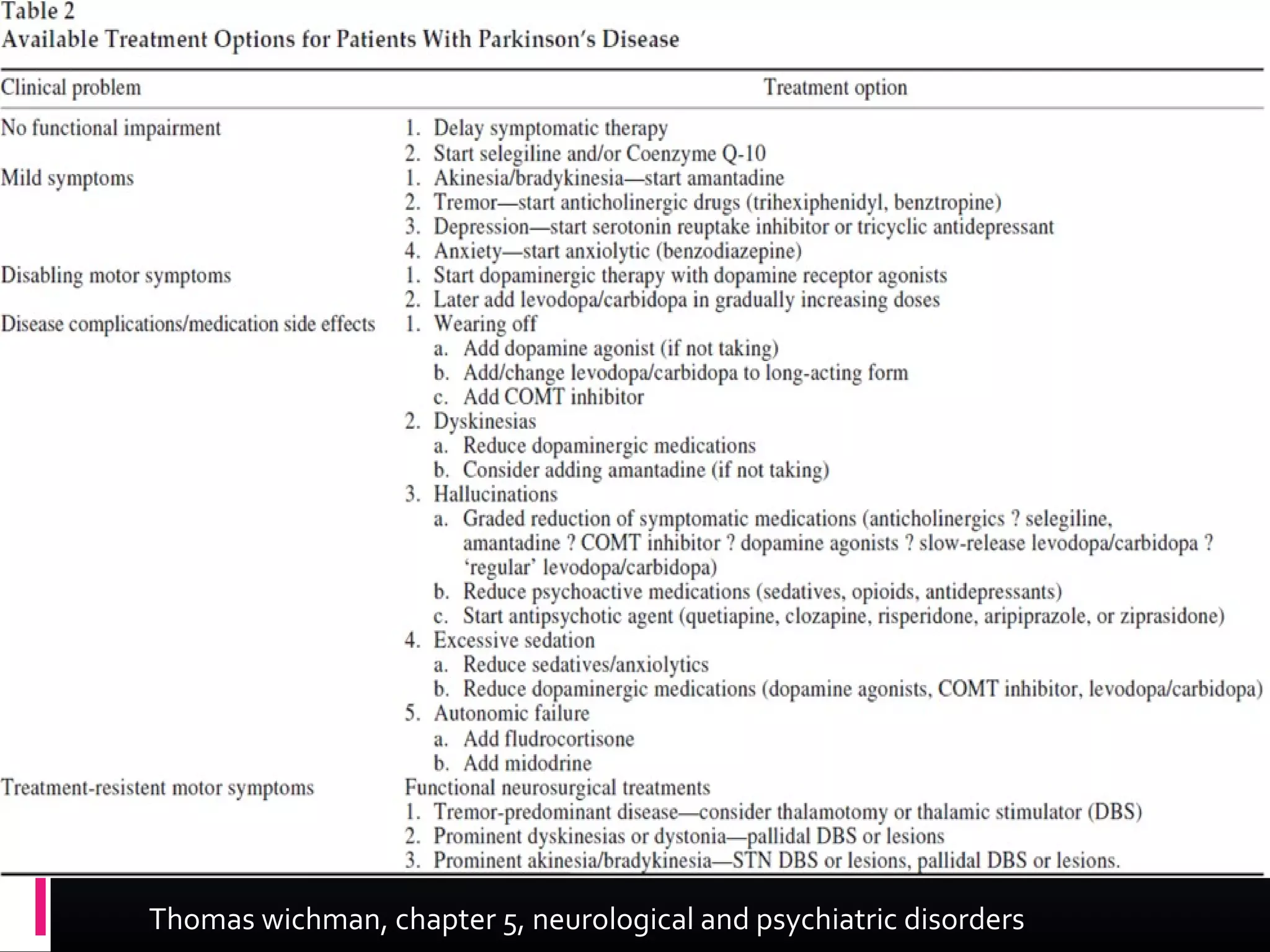
The document outlines various movement disorders in old age, particularly focusing on Parkinson's disease and its treatment options, including pharmacological, non-pharmacological, and surgical methods. It discusses the biochemical causes of Parkinsonism, the importance of early treatment initiation to maintain quality of life, and the various medications and therapies available to manage symptoms. Additionally, it highlights ongoing research into neuroprotective therapies, surgical treatments like deep brain stimulation, and addresses non-motor symptoms commonly associated with Parkinson's disease.


![Causes of parkinsonism
Idiopathic PD- 85% of all PS
Familial (hereditary) PD
Parkinsonian syndromes:
PSP- 1.5%
MSA- 2.5%
- Striato-NigralType
(Parkinsonism First)
- Shy-Drager Syndrome
(Autonomic Failure First)
- Olivo-ponto-cerebellarType
(OPCA – Ataxia First)
Corticobasal degeneration (CBD)
Secondary parkinsonism
Vascular- 3%
Drug induced- 7-9%
Postencephalitic
Hydrocephalus
Degenerative disorders with
associated parkinsonism:
Alzheimer disease (AD)
Parkinson—dementia—motor
neuron disease complex
Genetic disorders with associated
parkinsonism:
Wilson disease (consider in all
cases <50 years)
Huntington disease (HD)
(akinetic rigid [Westphal]
variant)
Dopa-responsive dystonia](https://image.slidesharecdn.com/managementofmovementdisorders-151013180529-lva1-app6892/75/Management-of-movement-disorders-3-2048.jpg)