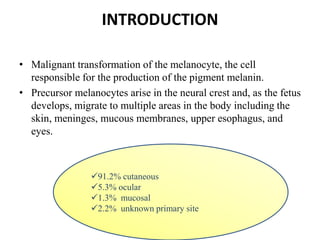
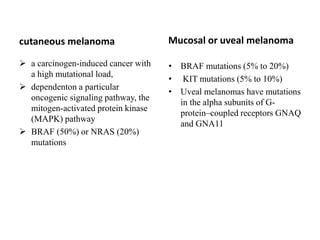
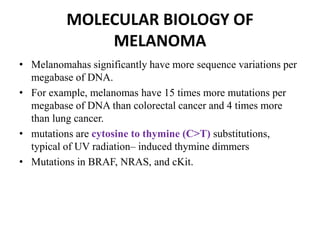
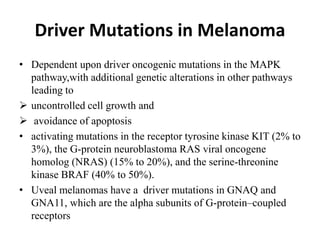
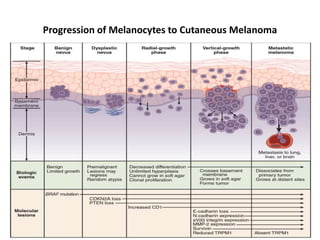
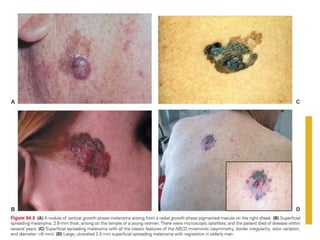
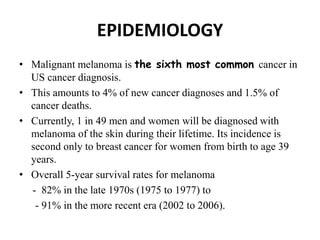
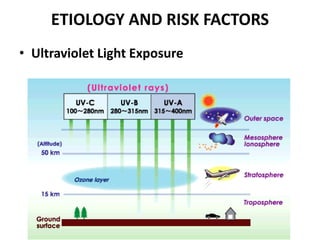
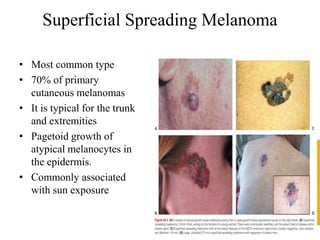
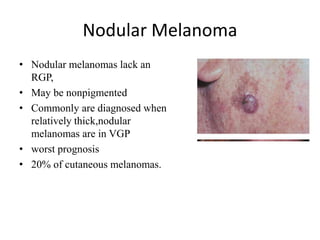
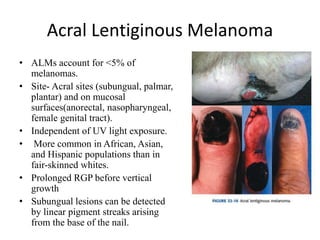
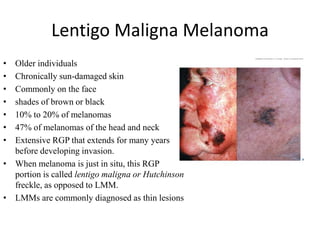
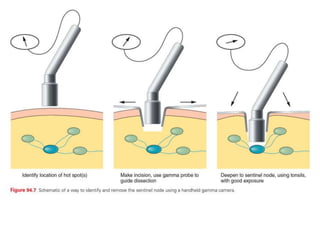
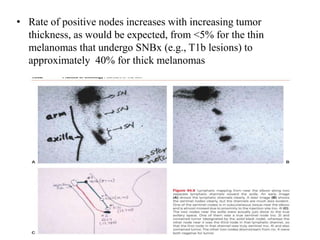
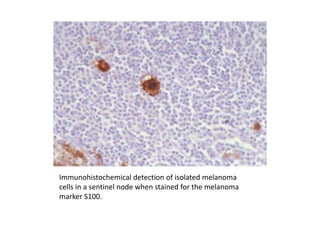
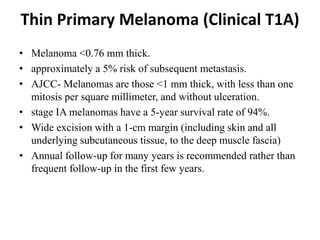
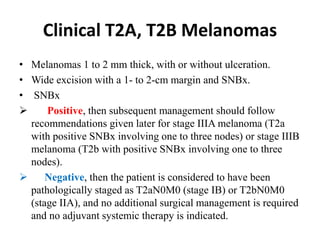
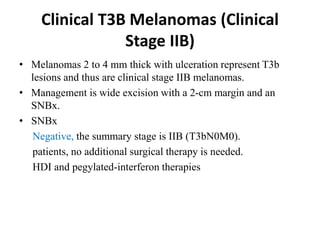
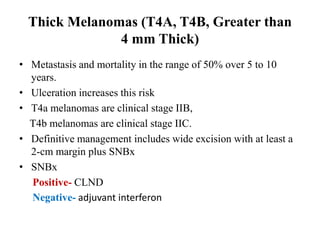
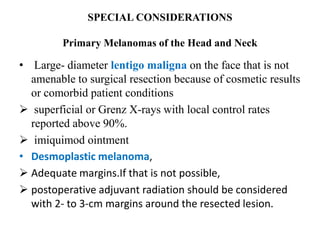
Malignant melanoma arises from melanocytes and most commonly presents on the skin. The document discusses the molecular biology, epidemiology, risk factors, diagnosis, histologic subtypes and prognosis of melanoma. It also covers management approaches including surgical excision and sentinel lymph node biopsy depending on tumor thickness and ulceration status. Positive lymph nodes indicate consideration of adjuvant therapies while negative nodes often do not require additional treatment.