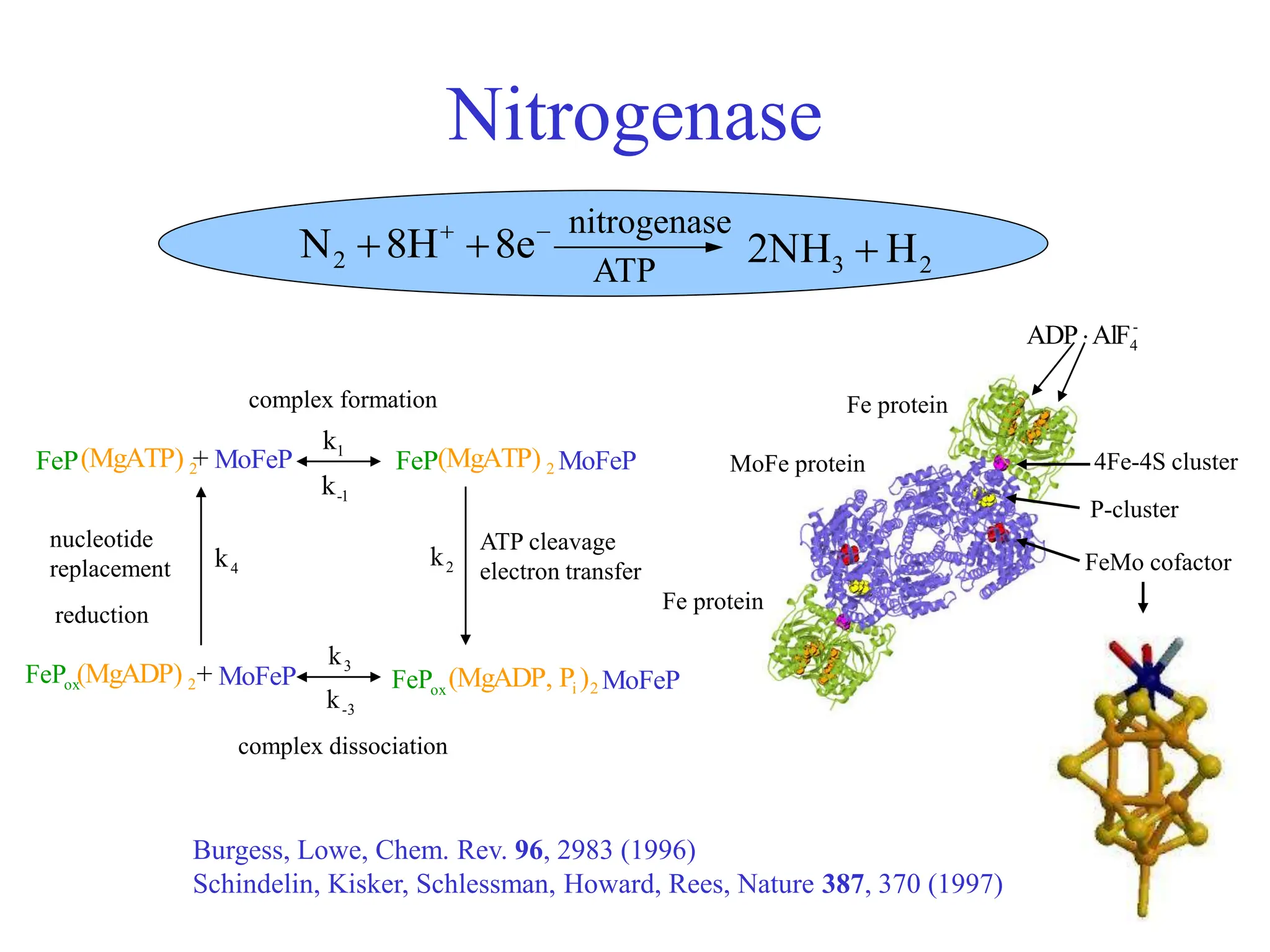
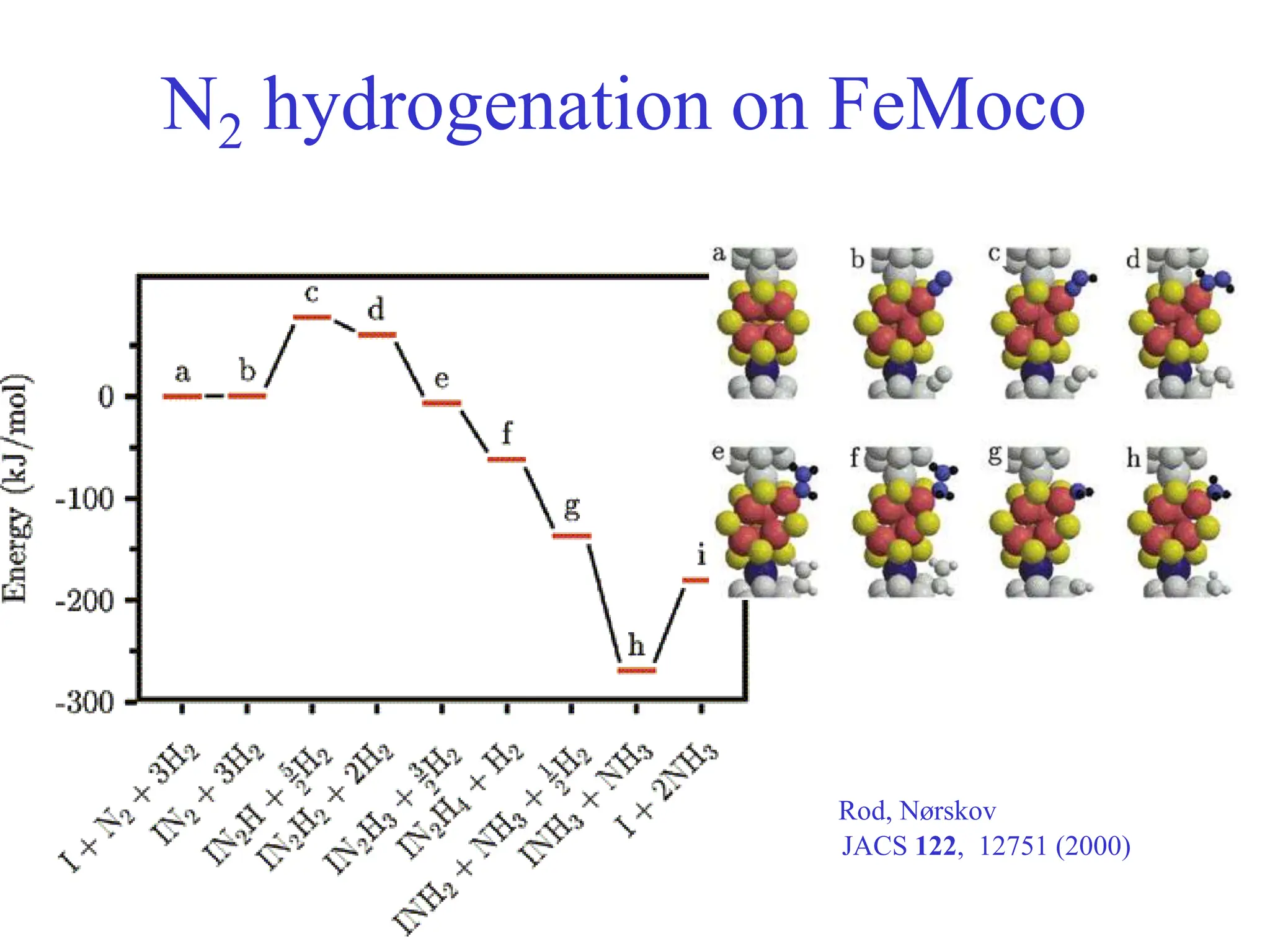
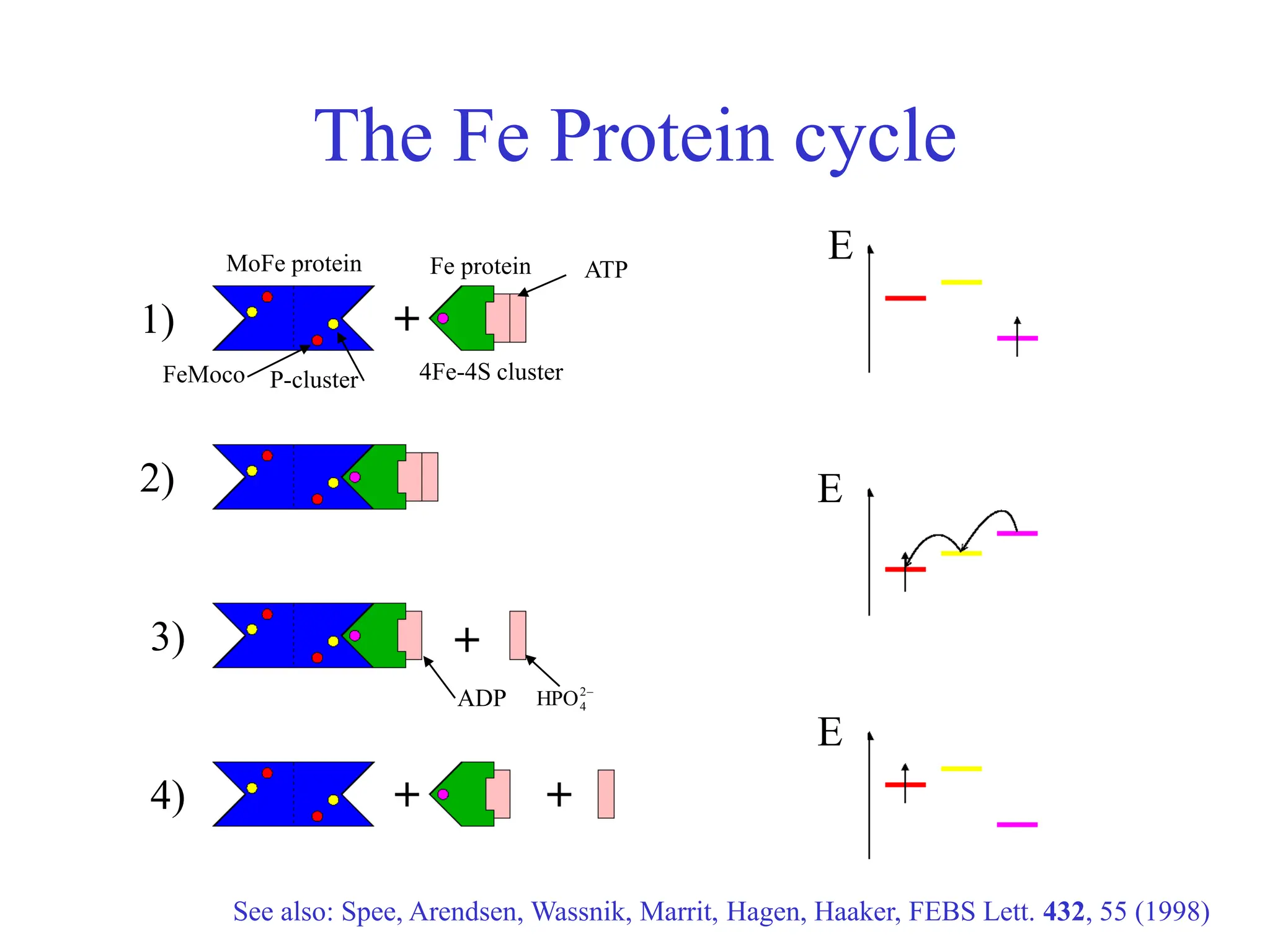
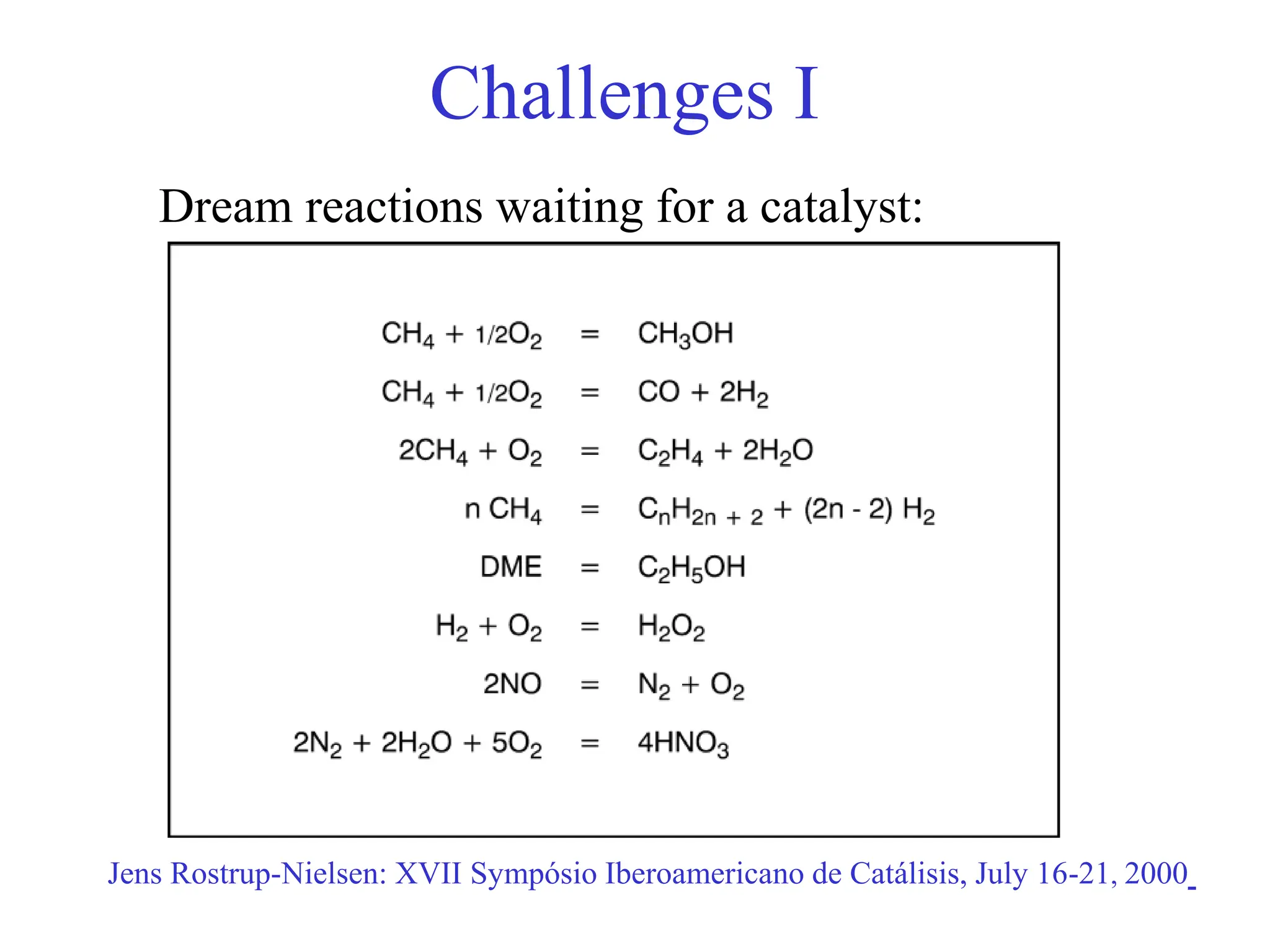
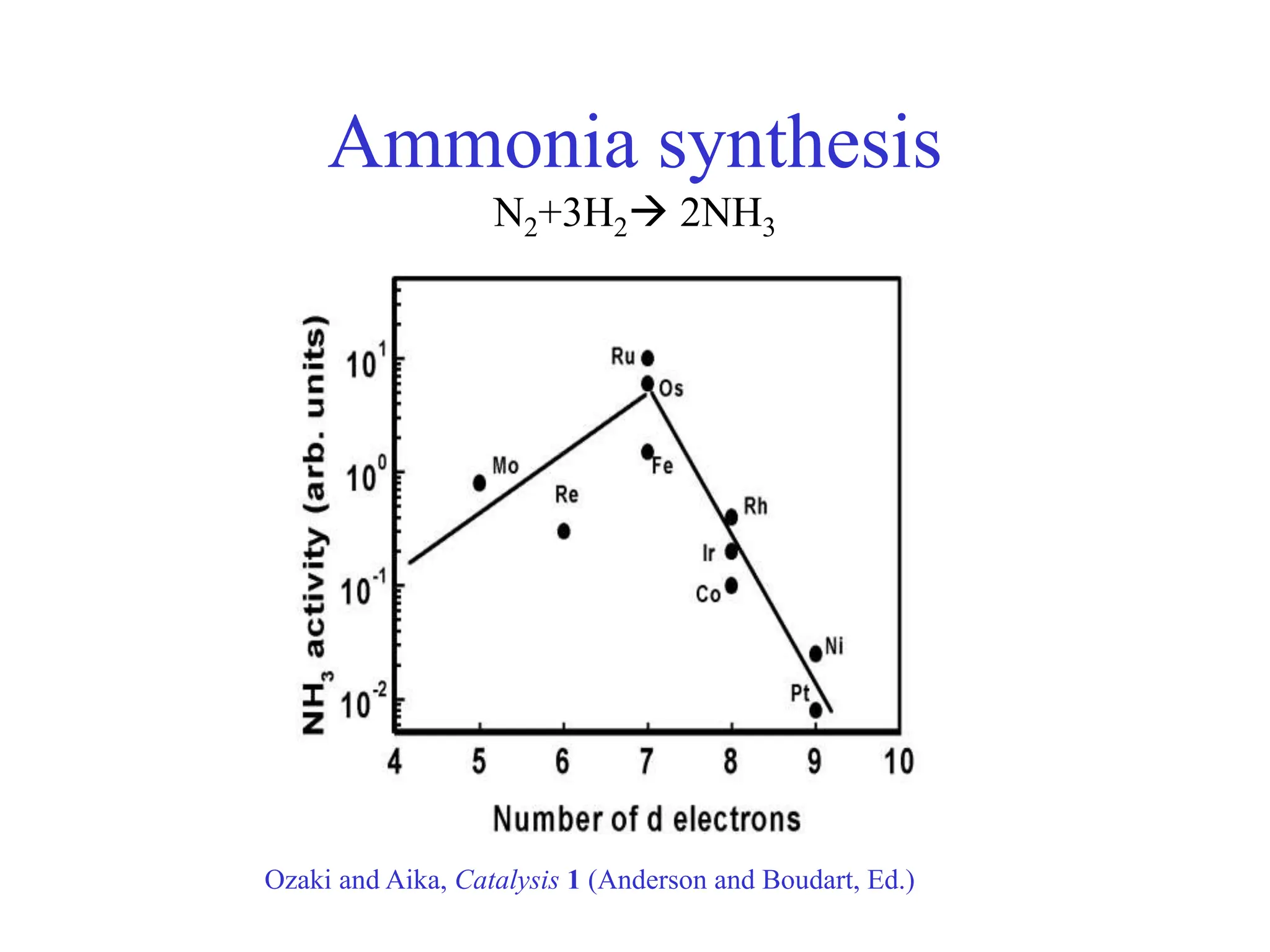
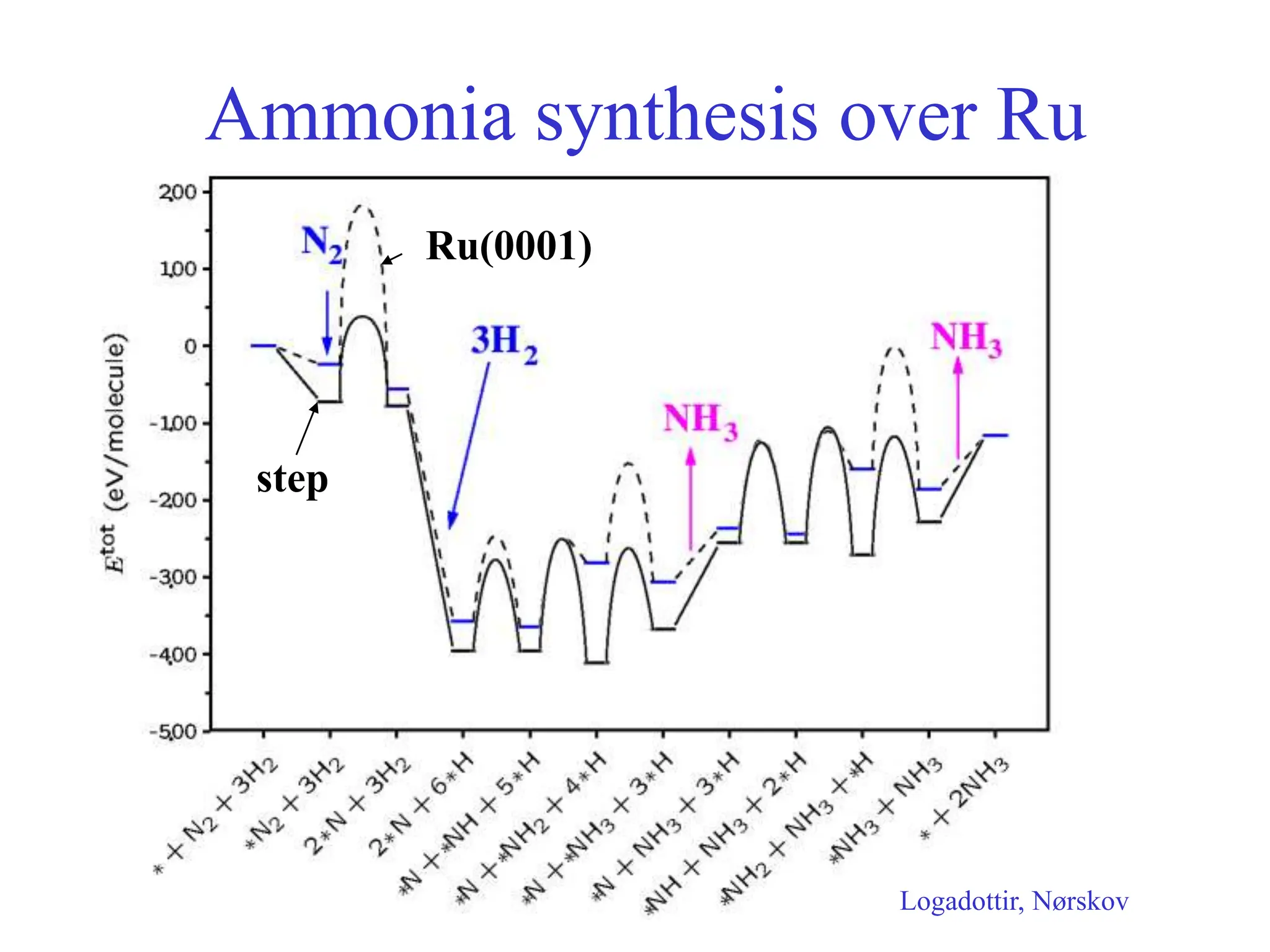
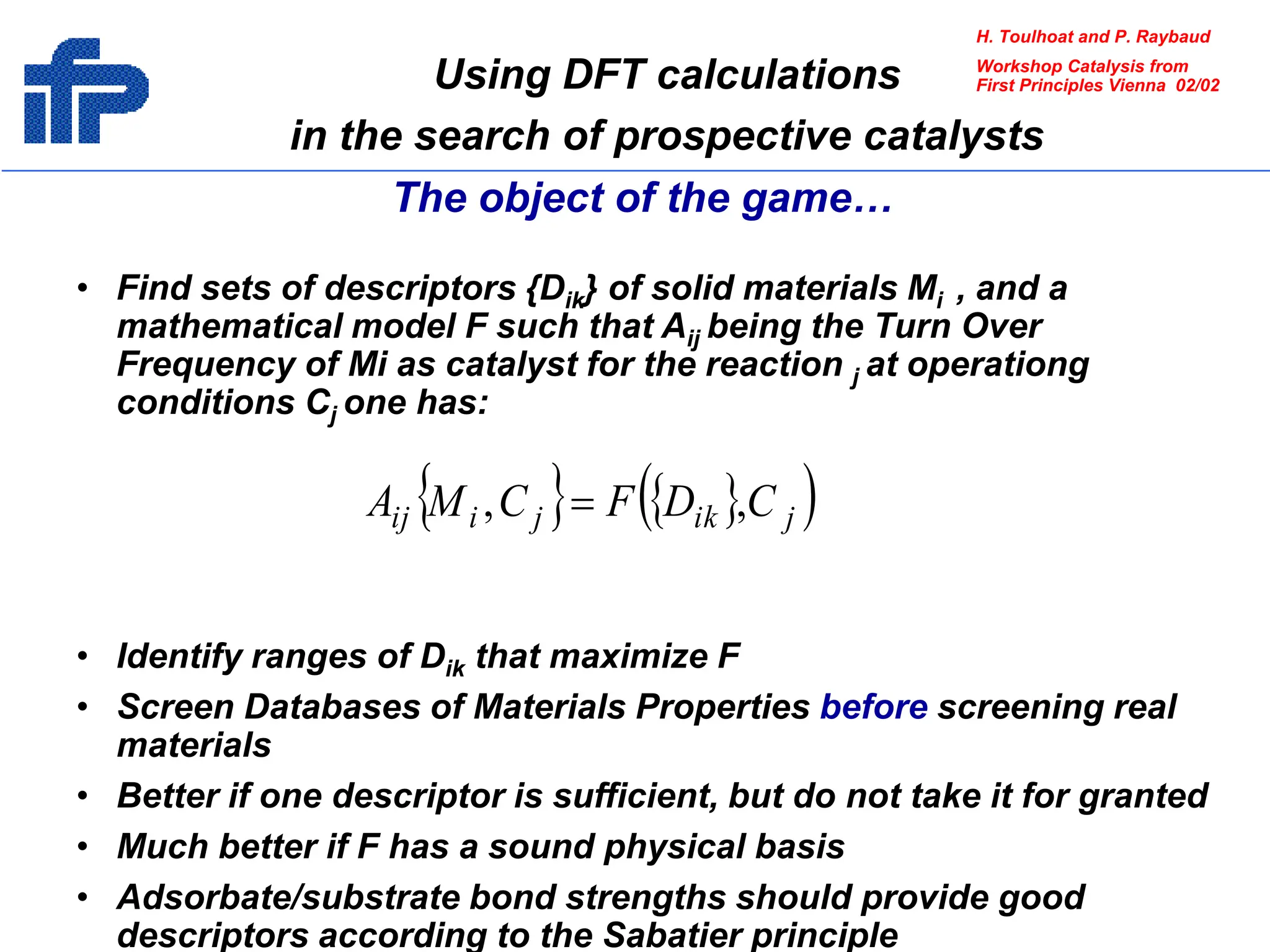
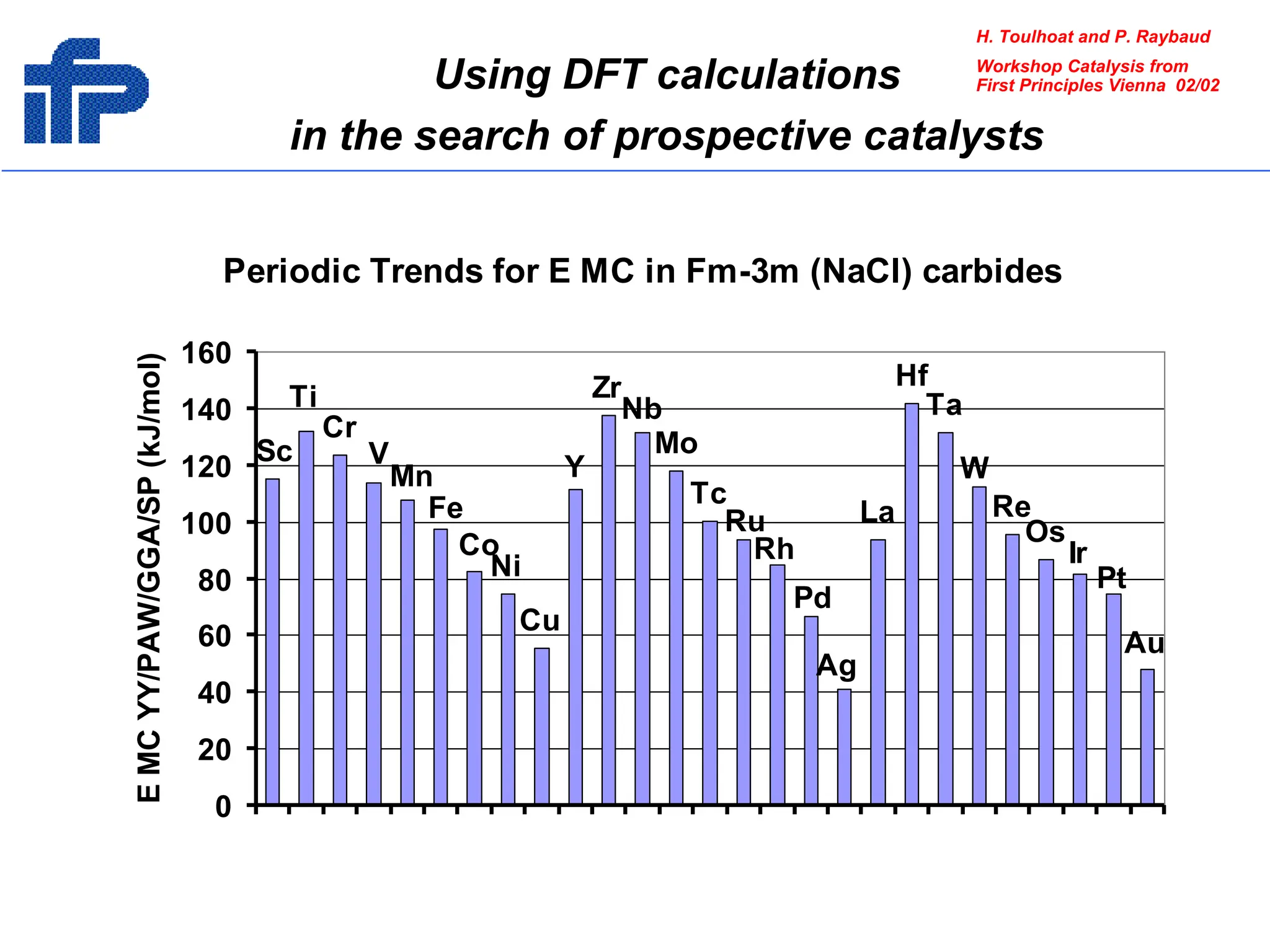
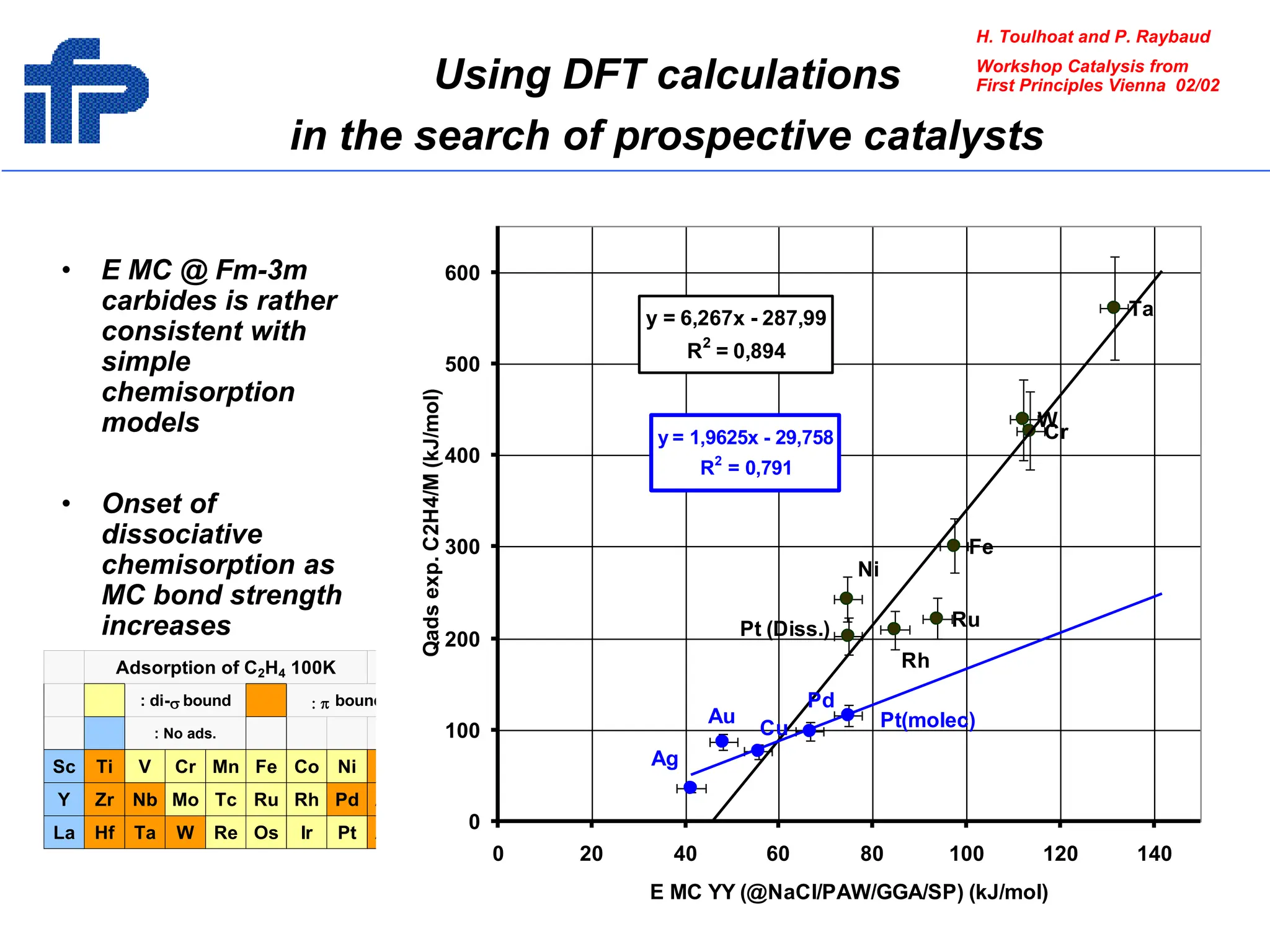
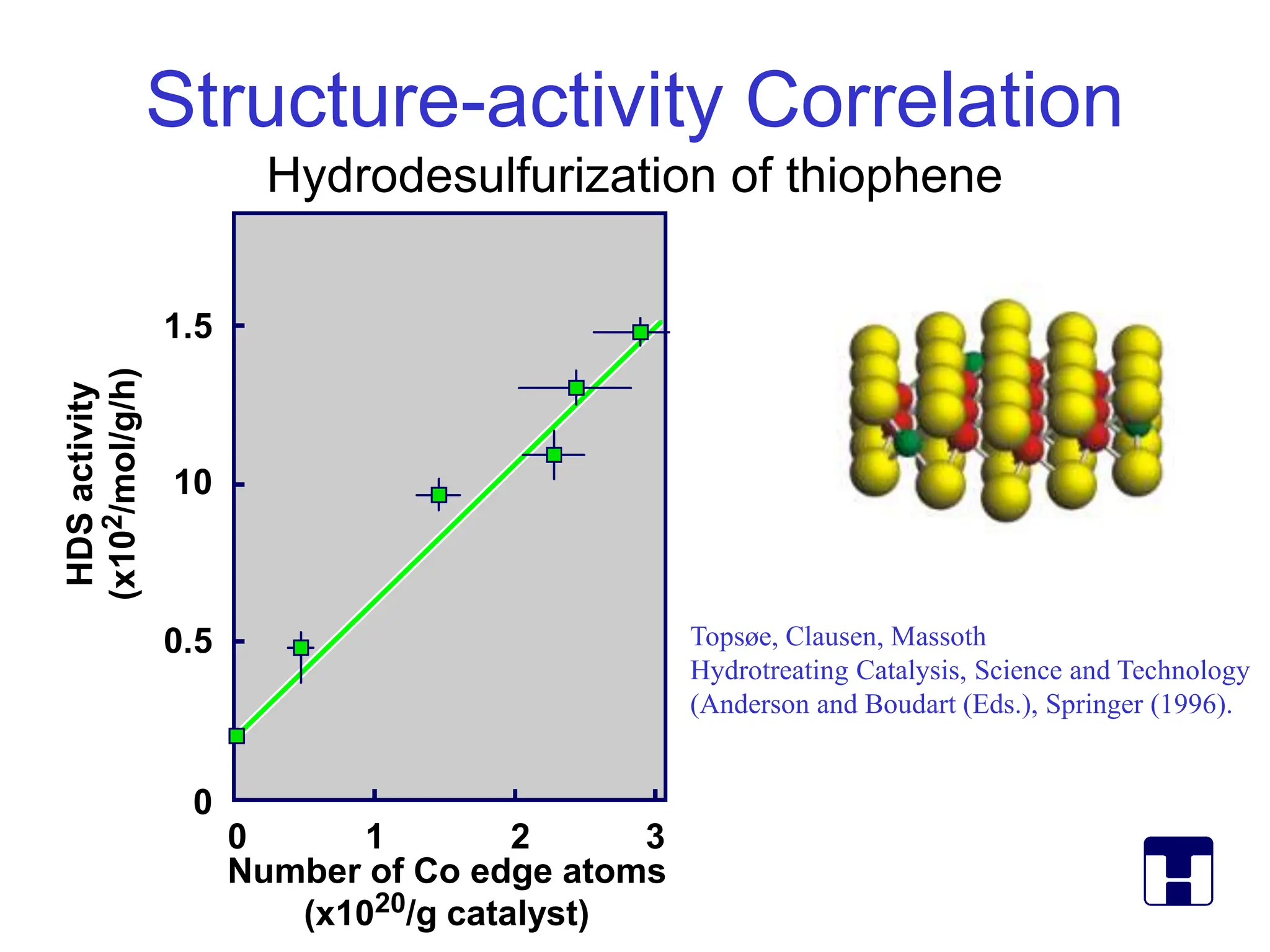
This document discusses opportunities and challenges in heterogeneous catalysis. The key challenges are meeting societal needs and developing a basic scientific understanding. Opportunities include designing catalysts at the nano-scale, rational catalyst design based on scientific insights, and accelerated discovery through large data-driven methods. Rational catalyst design requires understanding what determines catalytic properties and how to influence them. Basic understanding from computational and experimental studies is providing insights to guide catalyst optimization.



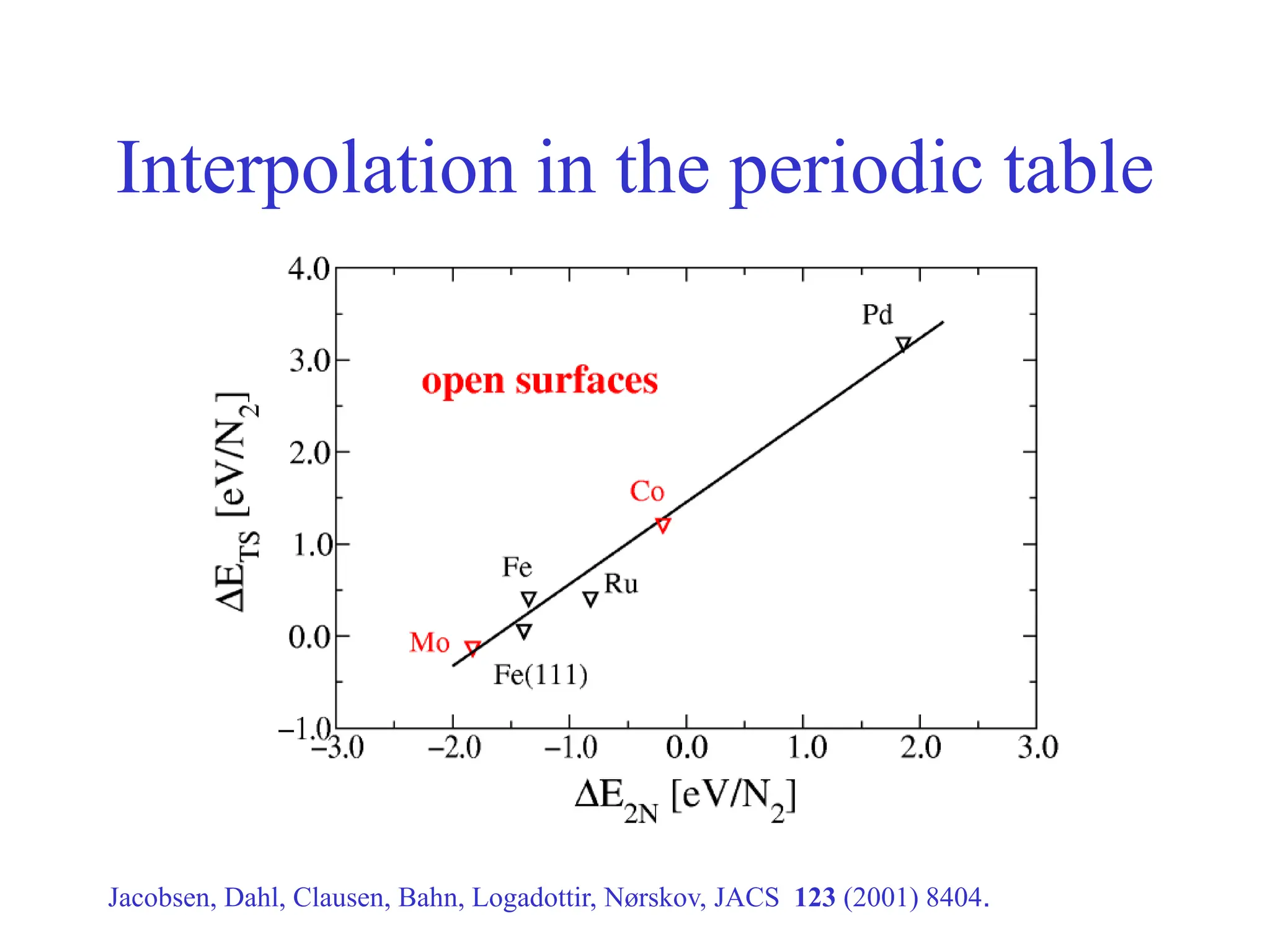
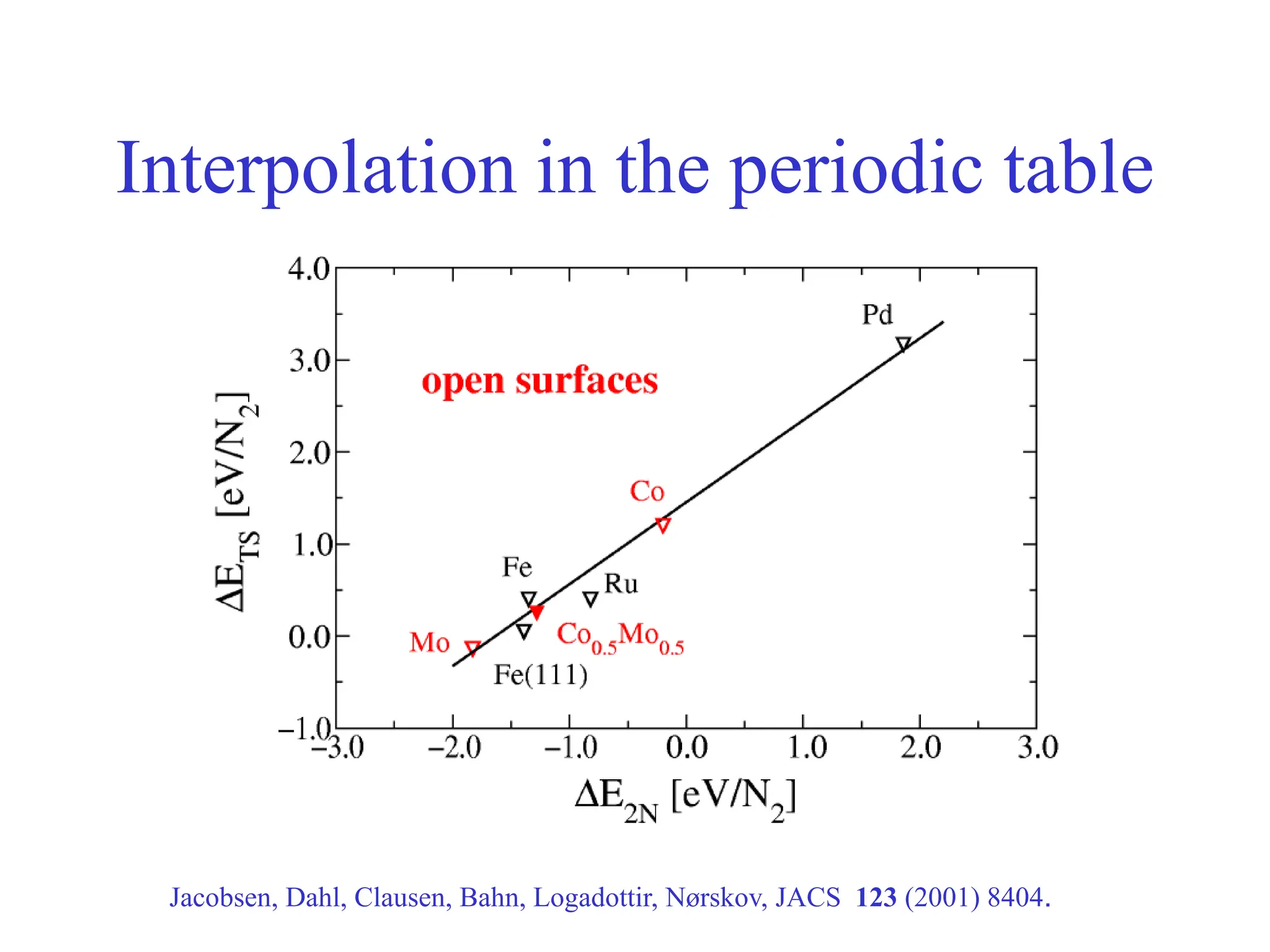
![-
0
.
8
-
0
.
40
.
00
.
40
.
8
[
E
-
E
(
R
u
)
]
(
e
V
/
N
2
)
1
0
-
5
1
0
-
4
1
0
-
3
1
0
-
2
1
0
-
1
1
0
0
1
0
1
T
O
F
(
s
-
1
)
F
e
M
o
R
u
C
o
N
i
O
s
Calculated ammonia synthesis rates
400 C, 50 bar, H2:N2=3:1, 5% NH3
Logatottir, Rod, Nørskov, Hammer, Dahl, Jacobsen, J. Catal. 197, 229 (2001)](https://image.slidesharecdn.com/catalysisnorskov0515021-240227111952-01b1d058/75/Heterogeneous-Catalysis-Opportunities-and-Challenges-11-2048.jpg)




![Single crystal microcalorimerty
Metal coverage [ML]
0 1 2 3 4 5 6 7 8
Heat
of
adsorption
[kJ/mol]
0
50
100
150
200
250
300
350
400
Cu/MgO
Ag/MgO
Pb/MgO
Larsen, Starr, Campbell, Chem.Thermodyn. 33, 333 (2001)
Brown, Kose, King, Chem. Rev. 98, 797 (1998).](https://image.slidesharecdn.com/catalysisnorskov0515021-240227111952-01b1d058/75/Heterogeneous-Catalysis-Opportunities-and-Challenges-23-2048.jpg)
![Methane activation on Ni/Ru
Ni Coverage [ML]
0 1 2
Initial
sticking
probability
0
1e-7
2e-7
3e-7
4e-7
5e-7
Thermal dissociation of CH4
at T = 530 K
Egeberg, Chorkendorff, Catal. Lett. 77, 207 (2001)](https://image.slidesharecdn.com/catalysisnorskov0515021-240227111952-01b1d058/75/Heterogeneous-Catalysis-Opportunities-and-Challenges-28-2048.jpg)