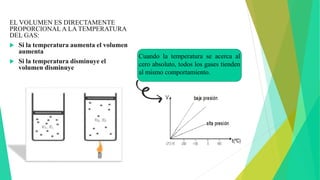
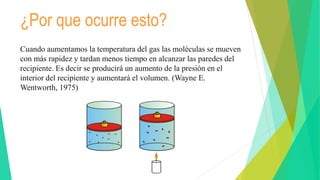
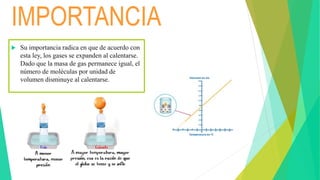
This document discusses Charles' law, which states that at constant pressure, the volume of a gas is directly proportional to its absolute temperature. It provides definitions and mathematical expressions of the law, and explains that it occurs because temperature is directly related to the kinetic energy and motion of gas molecules. Examples are given of how the law applies to hot air balloons, pressure cookers, and boiling milk. The document aims to explain Charles' law and its importance in relating the properties of gas volume and temperature.