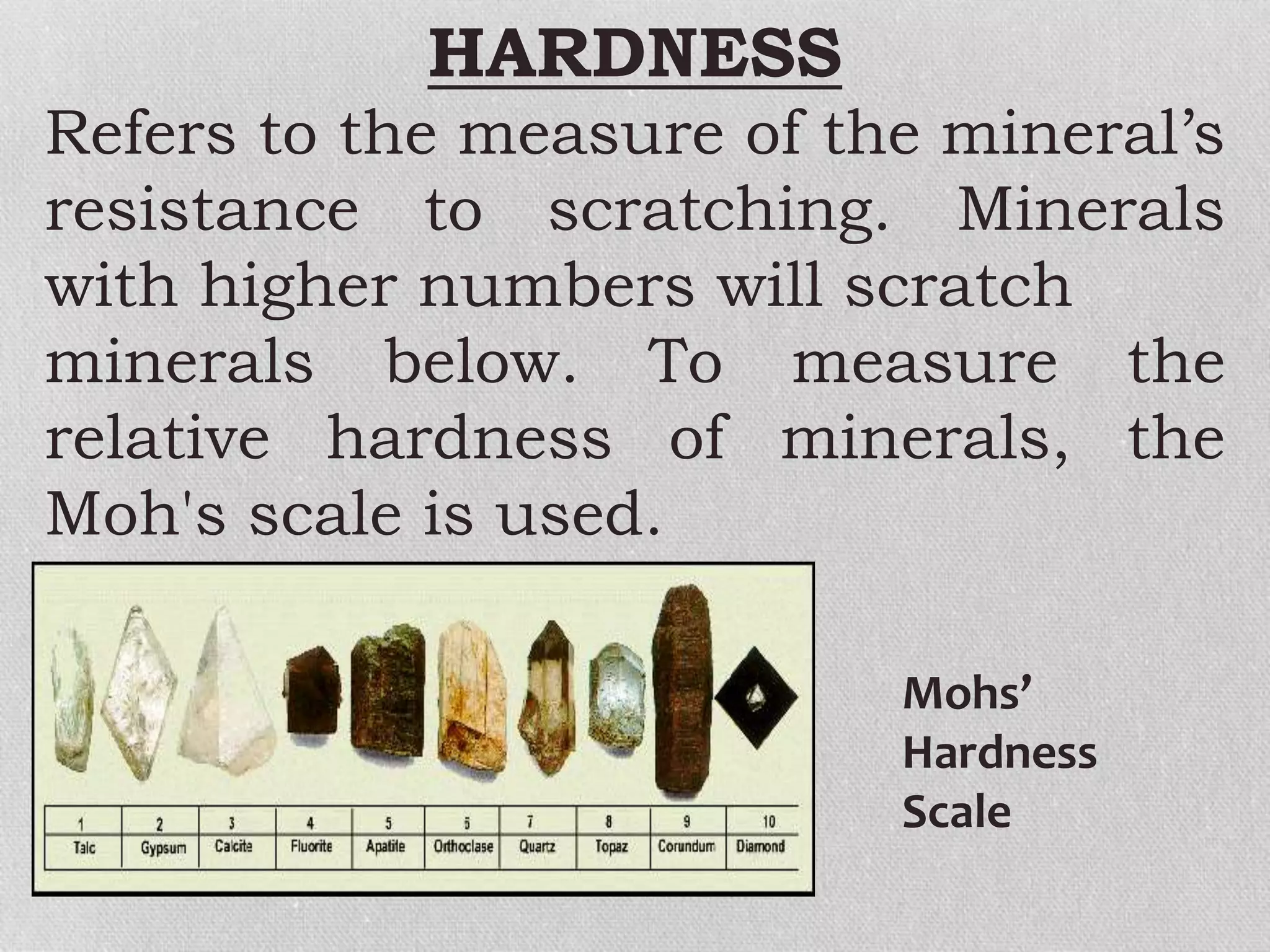
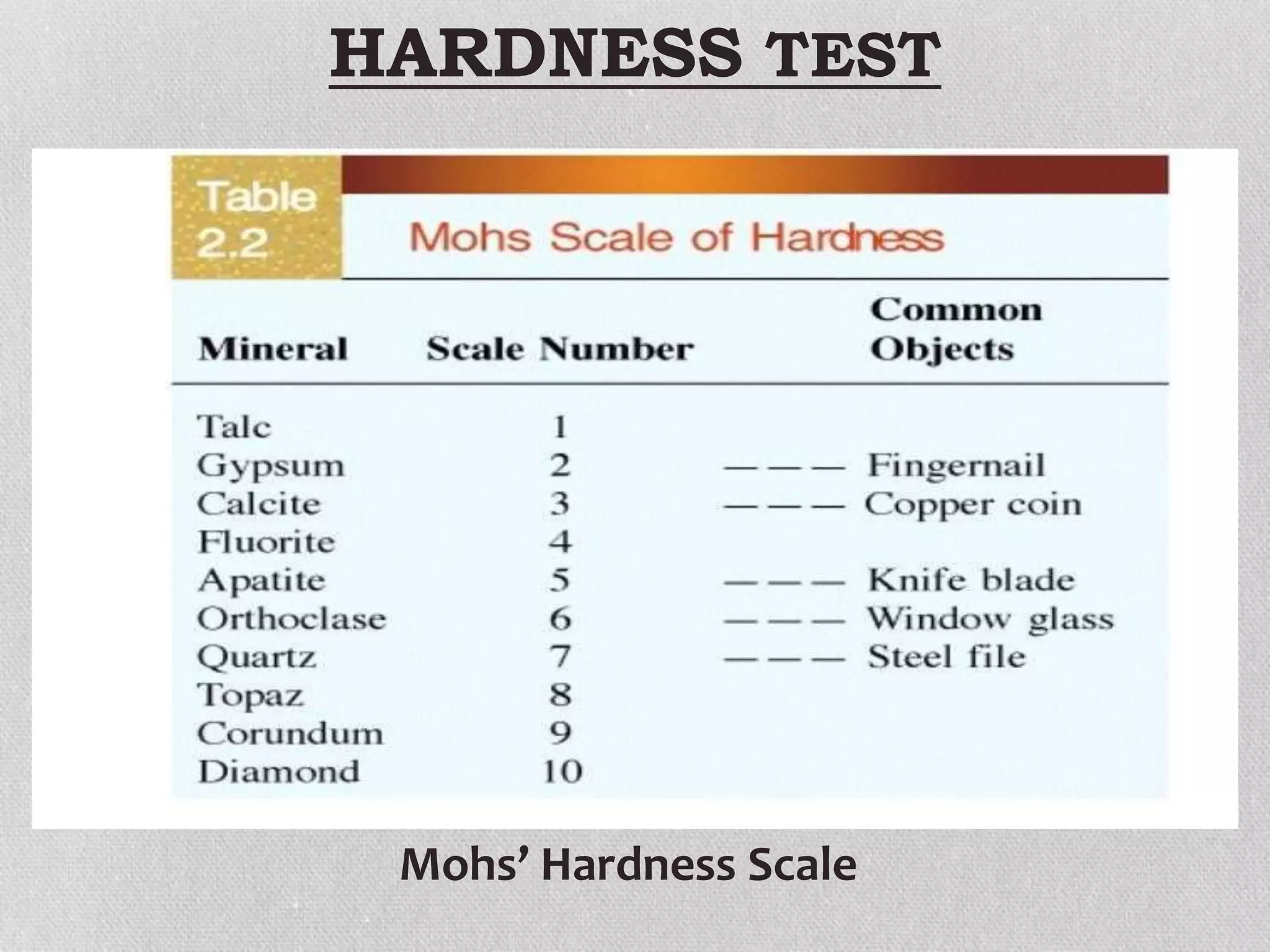
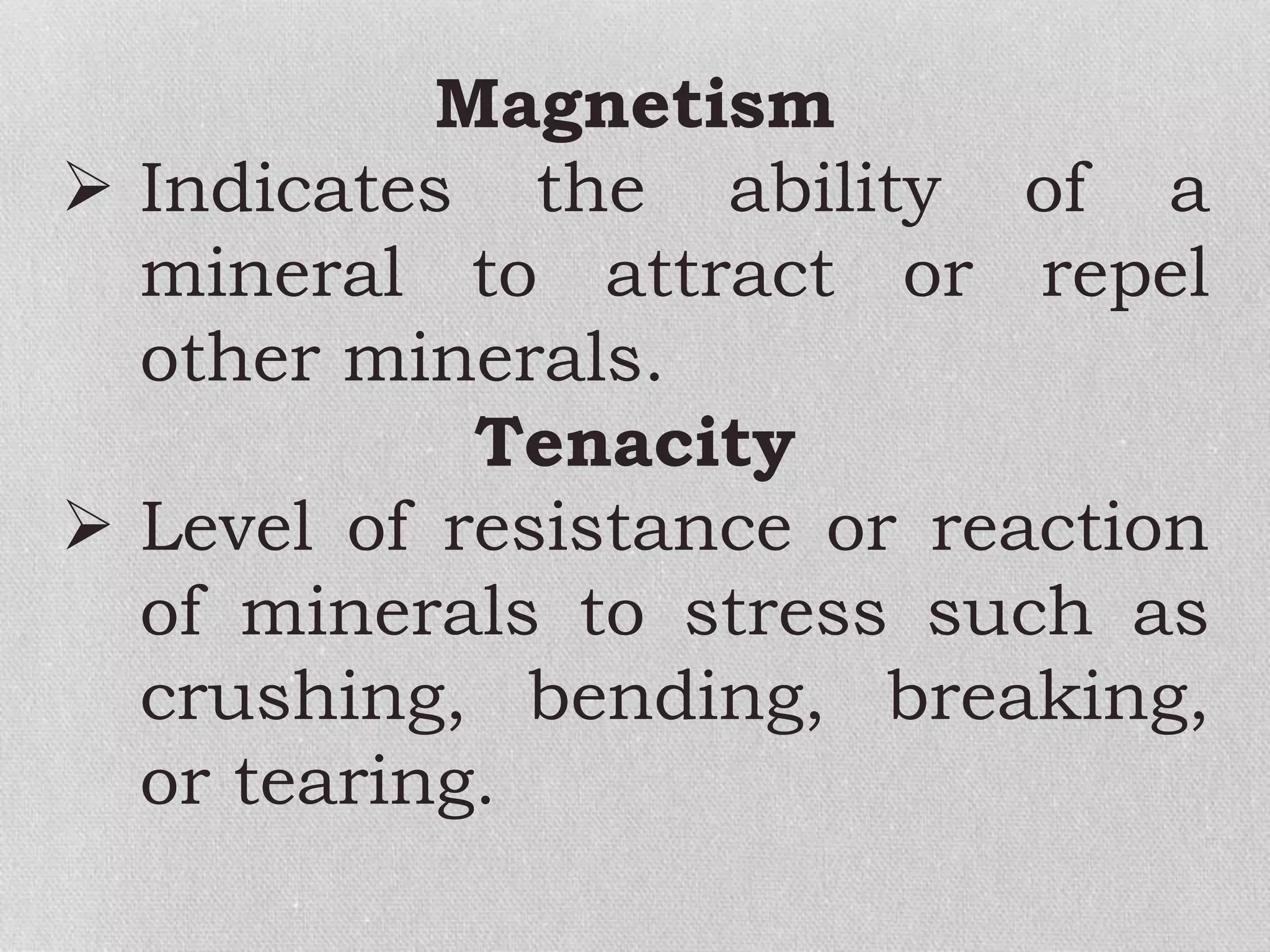
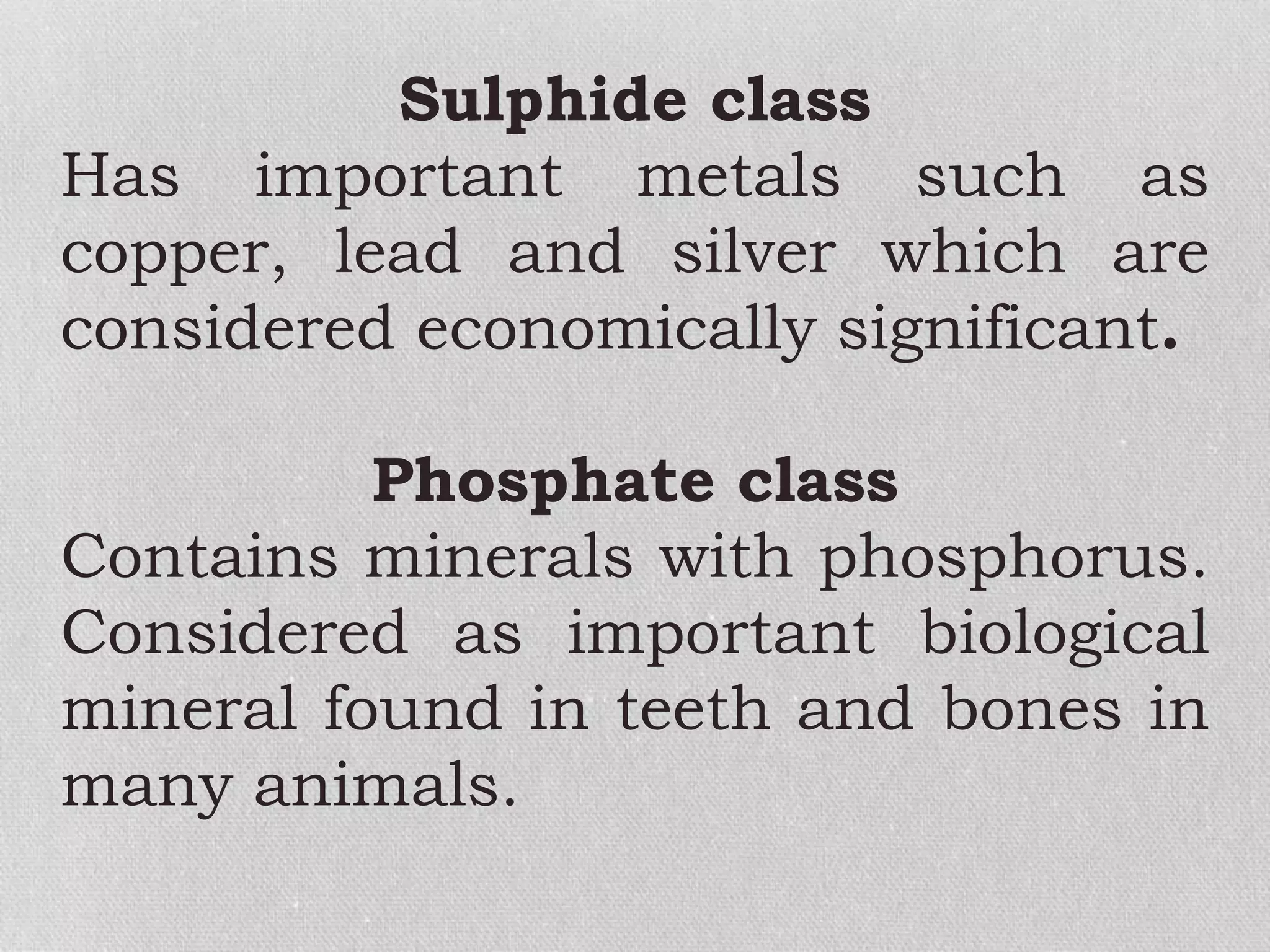
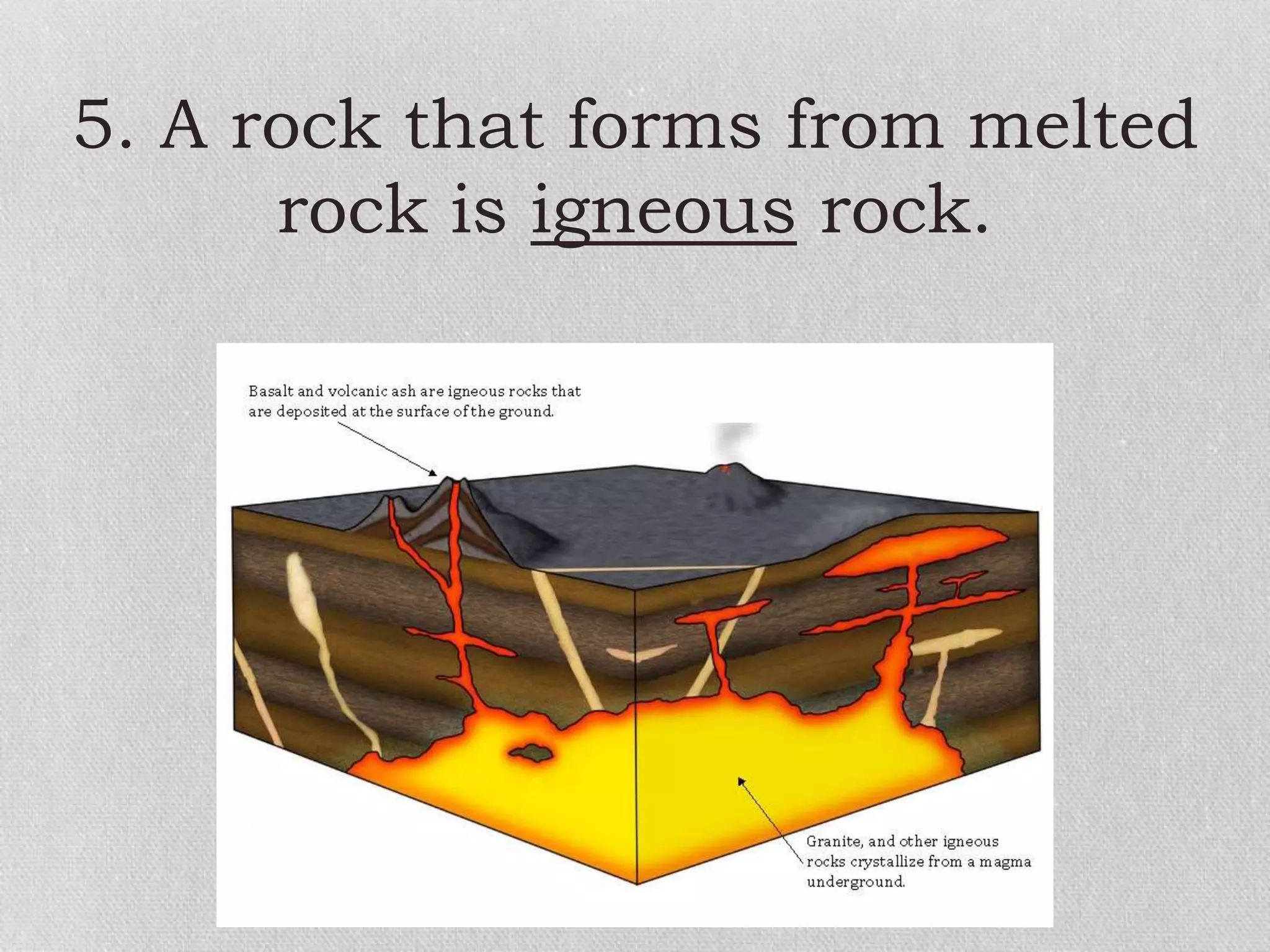
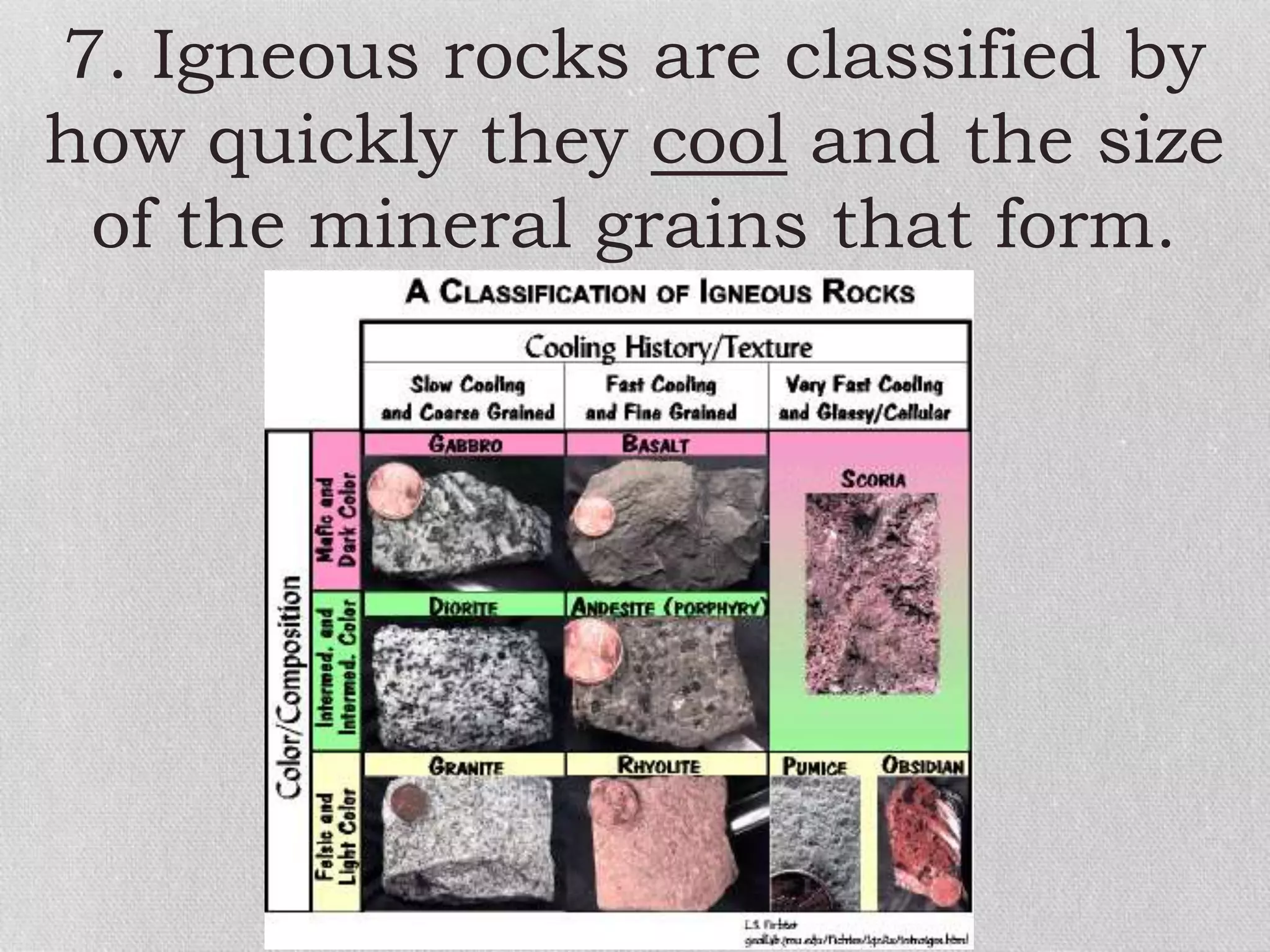
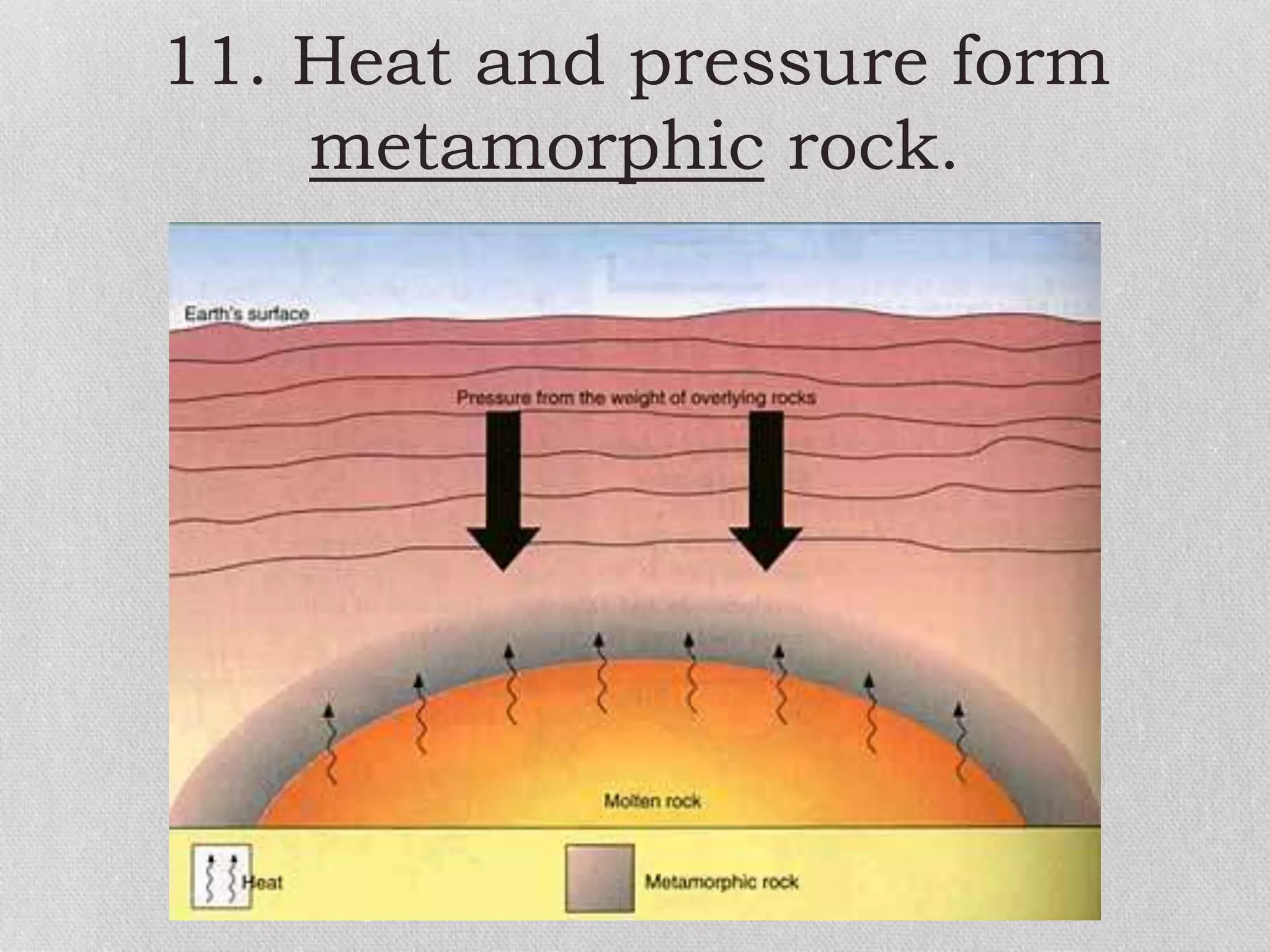
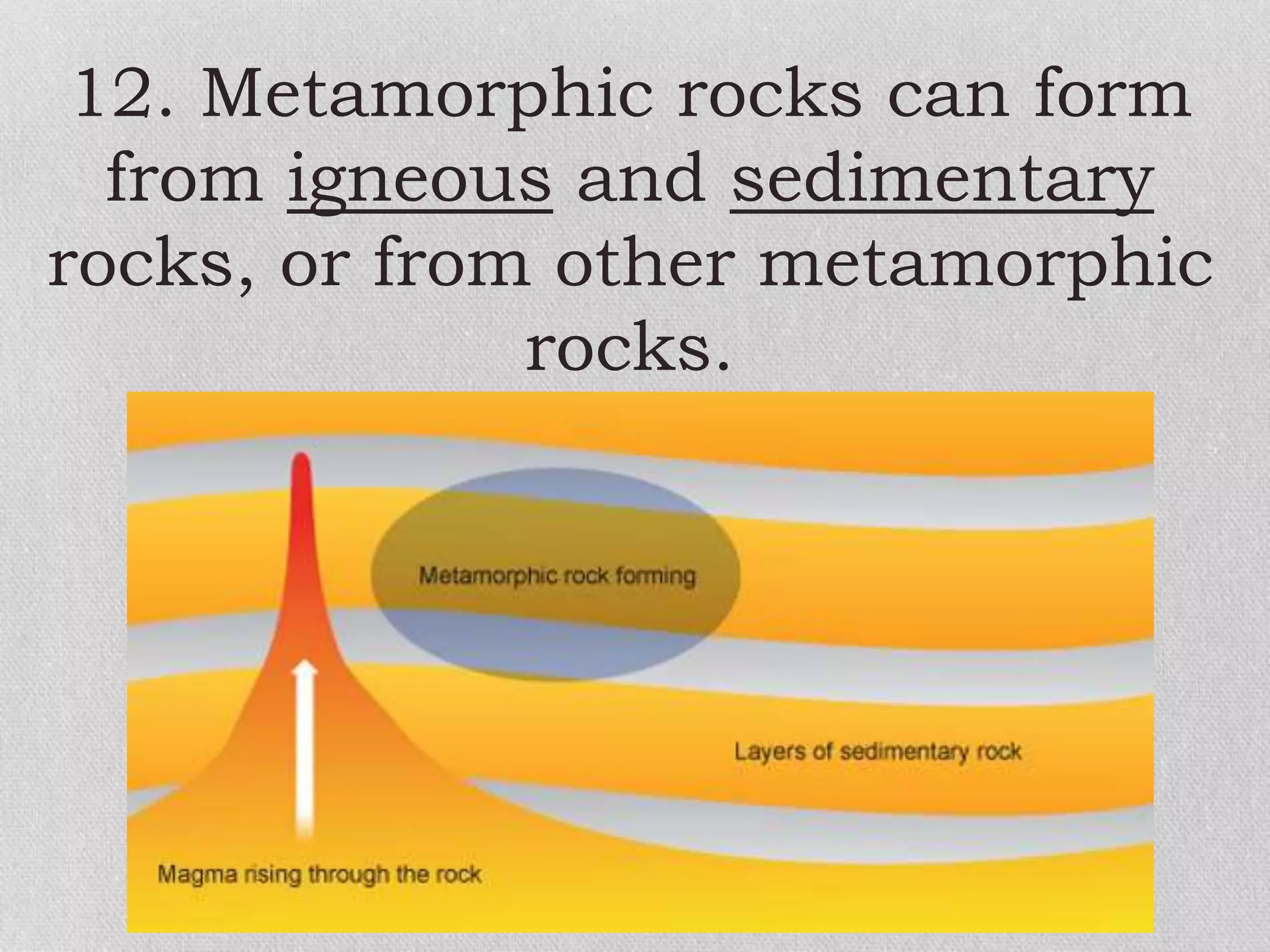
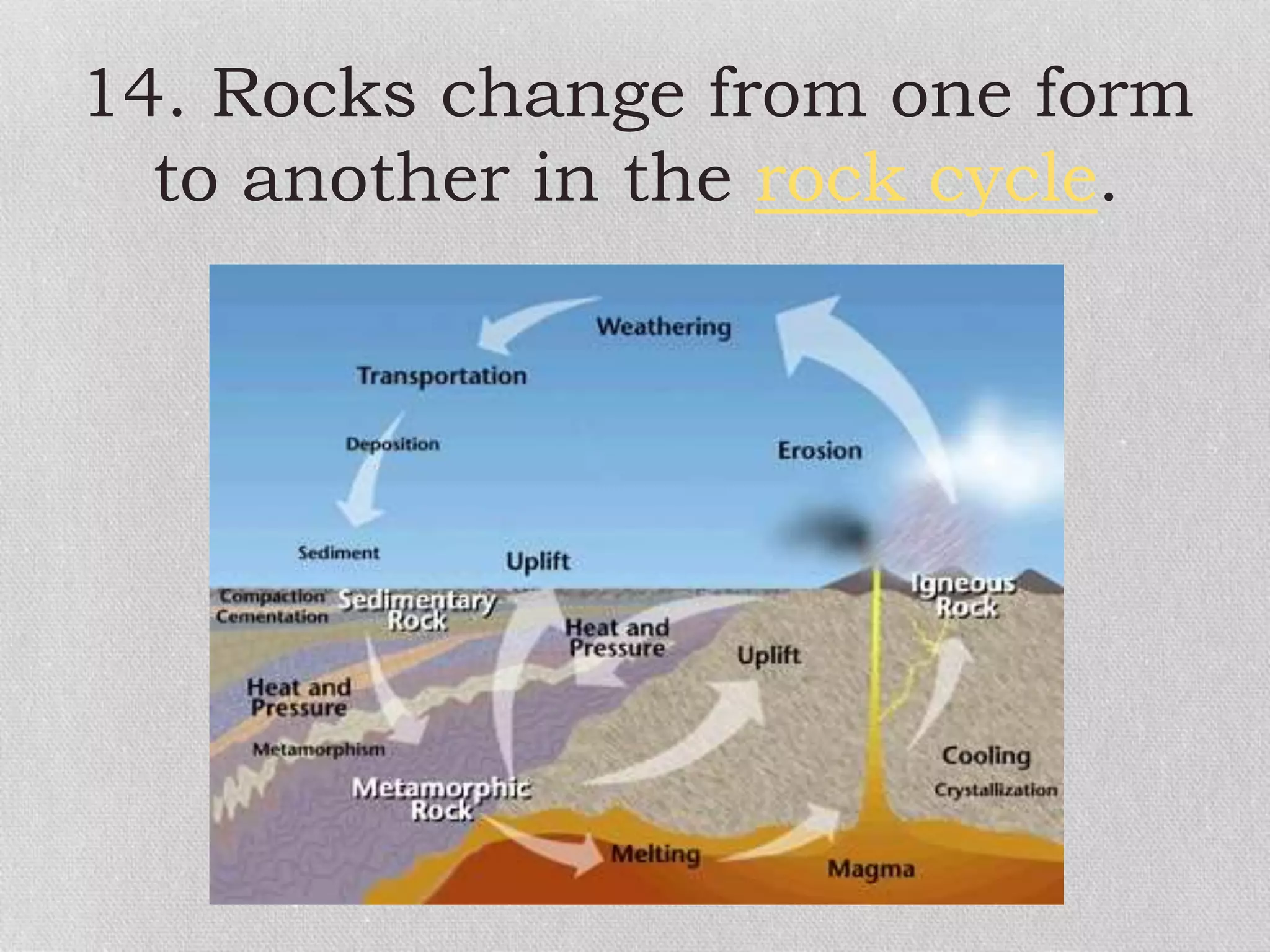
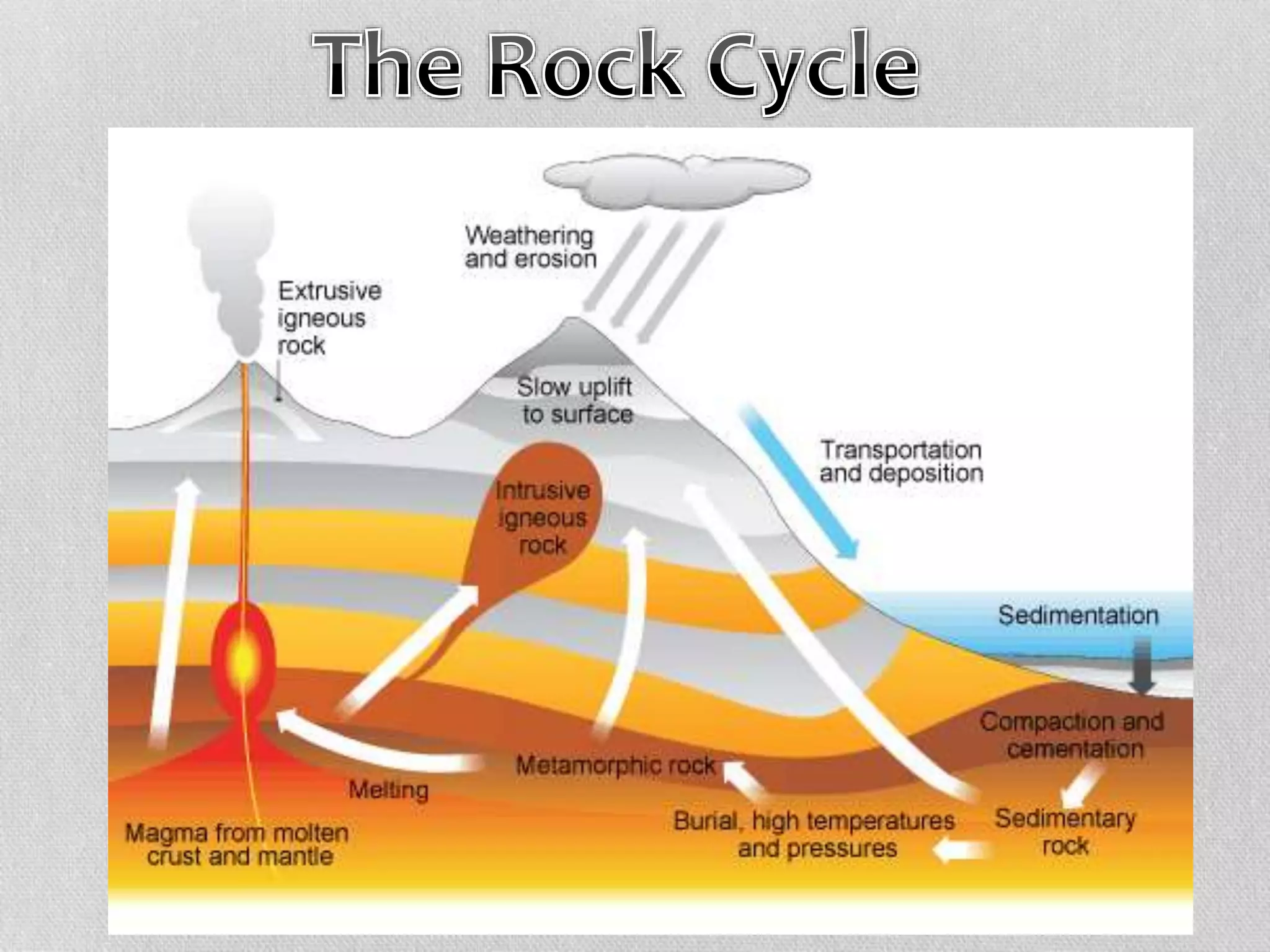
Minerals are naturally occurring, inorganic solids with a defined chemical composition and crystalline structure that make up rocks. The physical properties of minerals, such as color, hardness, streak, luster, cleavage, and crystal structure can be used to identify different types of minerals. Minerals are also classified based on their chemical composition into classes including silicates, carbonates, sulfates, halides, oxides, and sulfides. Understanding the physical and chemical properties of minerals provides clues about how the three main types of rocks - igneous, sedimentary, and metamorphic - form.