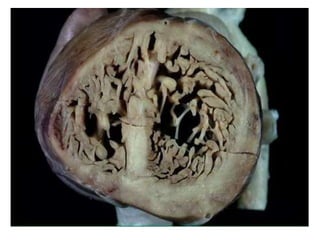
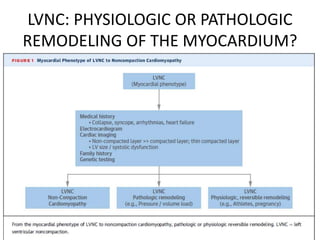
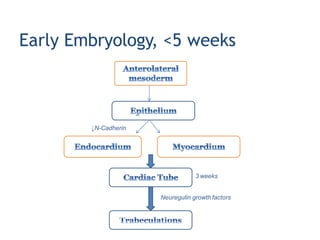
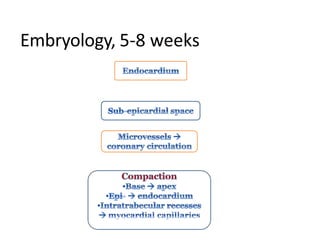
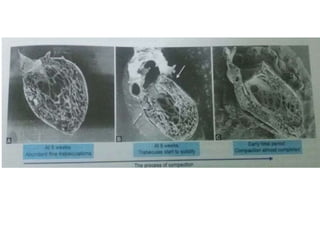
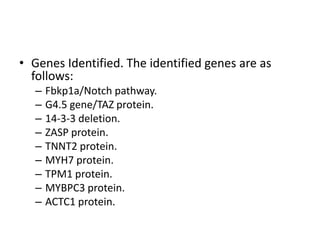
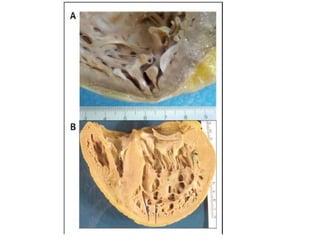
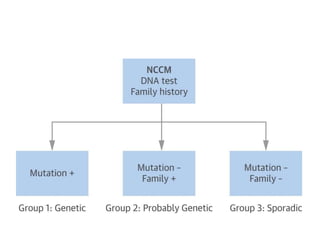
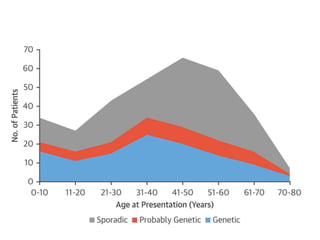
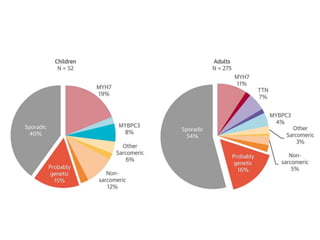
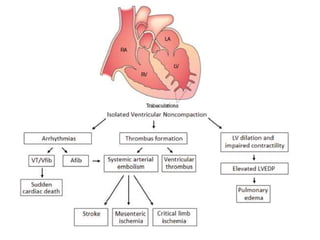
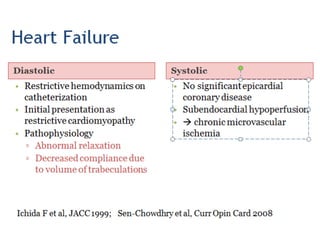
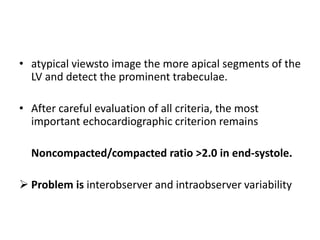
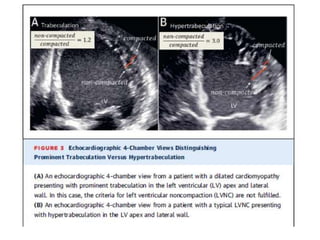
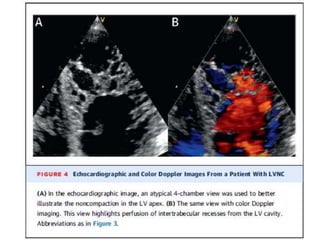
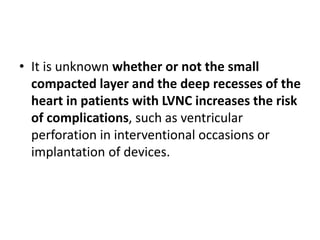
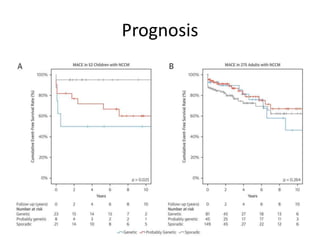
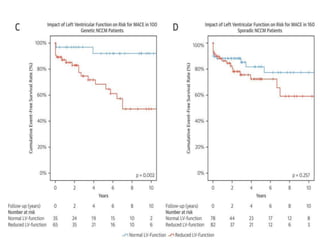
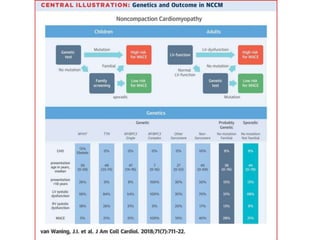
Left ventricular noncompaction (LVNC) is a rare cardiomyopathy characterized by prominent trabeculations and deep recesses in the left ventricular wall. It results from the failure of embryonic myocardial compaction. LVNC can be isolated or associated with other conditions. The diagnosis is made using echocardiography or cardiac MRI based on specific criteria. Management involves treating any heart failure, arrhythmias, or thromboembolic risks present. While genetic causes have been identified in some families, the underlying pathogenesis remains incompletely understood and LVNC remains a diagnostic and management challenge.