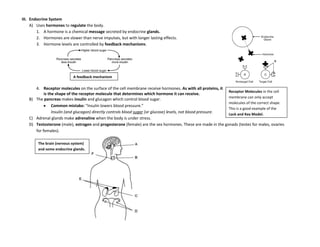
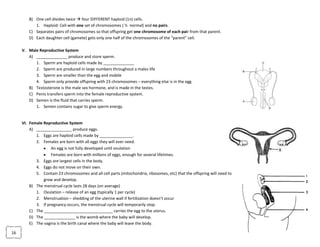
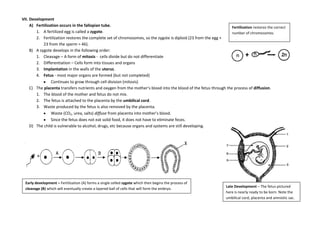
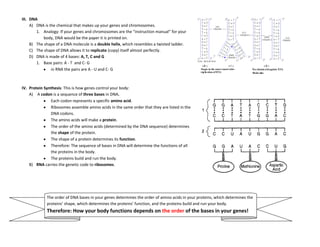
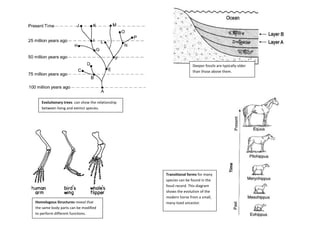
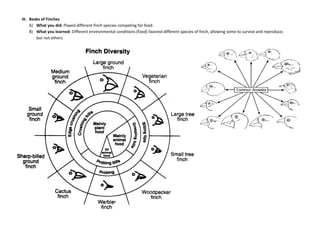
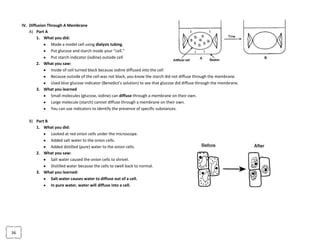
The document provides an overview of the Regents Exam for Living Environment in New York State. It is broken into four parts worth a total of 85 points. Part A covers general knowledge multiple choice, Part B applies knowledge through multiple choice and drawing graphs, Part C applies material to real world situations through short answer, and Part D pertains to labs performed during the school year through multiple choice and short answer. The exam covers 10 main topics including chemistry of living things, cells, nutrition/photosynthesis/respiration, the human body, reproduction, genetics, evolution, ecology, and state labs. Answers must be written in permanent pen without errors.