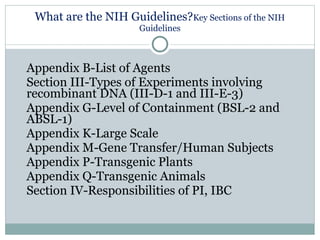
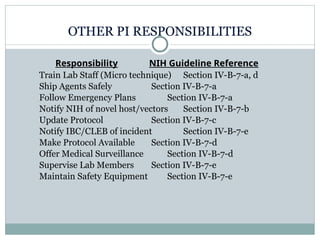
Research involving recombinant DNA is regulated by the NIH and requires a Biological Use Authorization (BUA) at UC Berkeley, overseen by the Committee on Laboratory & Environmental Biosafety (CLEB). Principal Investigators (PIs) must comply with NIH guidelines, conduct lab-specific biosafety training, and report any incidents involving recombinant DNA. Key elements include risk assessment, personal protective equipment, waste disposal, and emergency procedures to ensure laboratory safety.