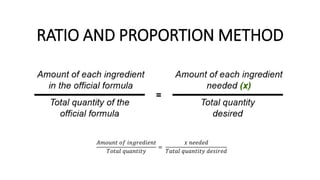
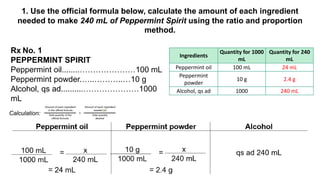
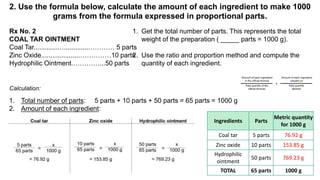
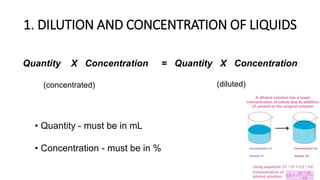
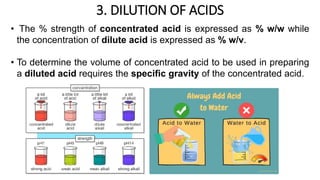
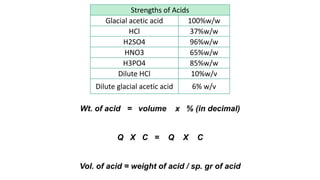
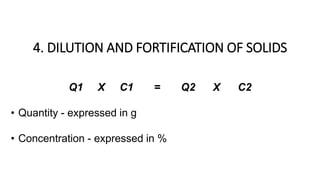
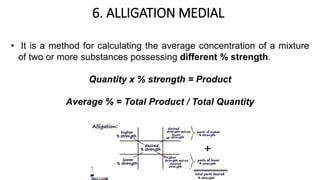
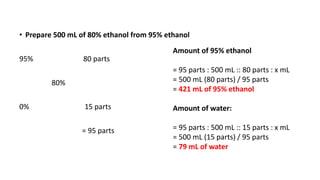
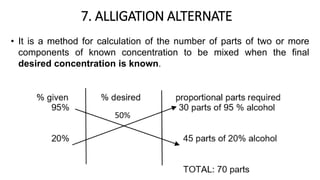
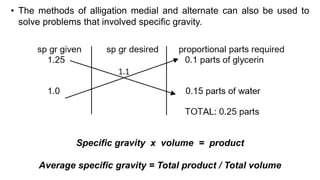
The document discusses the methods for altering the concentration of pharmaceutical preparations through various techniques such as dilution, fortification, and scaling formulas. It outlines two primary methods for adjusting ingredient quantities: ratio and proportion, and factor method, emphasizing the importance of maintaining correct ingredient proportions. Additionally, it covers calculations for preparing specific concentrations and strengths, including methods like trituration and alligation for achieving desired final concentrations.