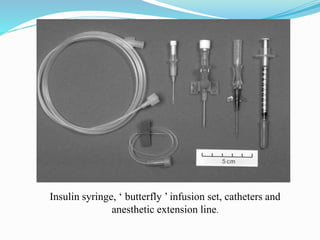
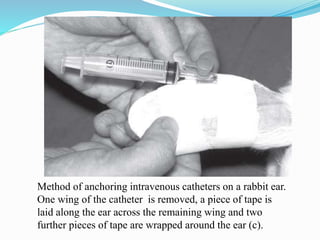
This document discusses anesthesia procedures for laboratory animals. It describes the types of animals commonly used in research, which are mostly rodents like mice and rats. It emphasizes the importance of pre-operative care and preparation of equipment, facilities, drugs and personnel. It categorizes different levels of anesthetic risk and discusses the physiological effects of anesthesia. It provides guidelines for fasting, dosing, injection equipment and monitoring anesthesia during procedures. The focus is on best practices for anesthesia to ensure humane treatment of research animals.