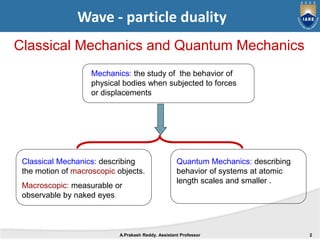
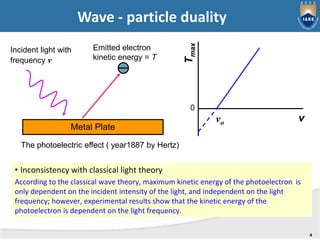
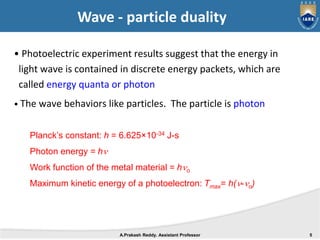
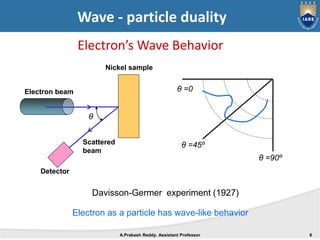
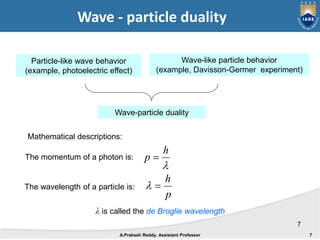
Quantum mechanics describes the behavior of matter at small scales. It explains phenomena like the photoelectric effect and black body radiation that classical mechanics could not. The document discusses key experiments that demonstrated the wave-particle duality of light and matter, such as the photoelectric effect showing light behaving as particles and the Davisson-Germer experiment showing electrons behaving as waves. Together, these experiments showed that quantum objects can exhibit both wave and particle properties, forming the basis of wave-particle duality central to quantum mechanics.