Crystalline solids have a regular, repeating arrangement of particles that gives them distinct properties including:
- Sharp melting points as they melt at specific temperatures
- Anisotropic properties that vary in different directions
Amorphous solids lack long-range order and instead have a random, unordered structure. As a result, they exhibit:
- A gradual softening and flowing over a temperature range without a distinct melting point
- Uniform properties in all directions
Some key examples of each are crystalline solids like NaCl, metals, and diamonds and amorphous solids such as glass, plastic, and rubber.






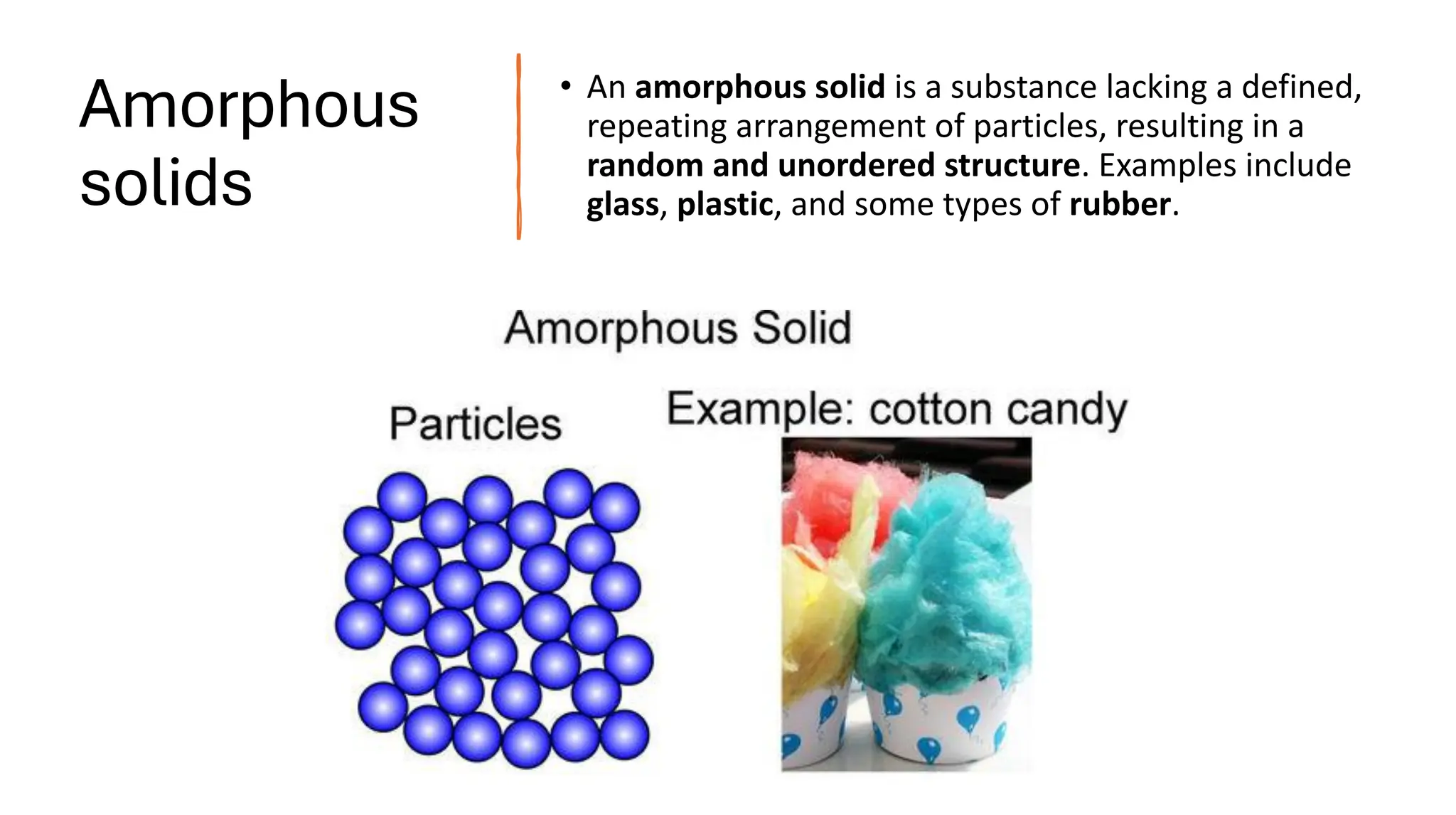

![Distinguish between crystalline solids and
Amorphous Solids. [13,14,17,19]](https://image.slidesharecdn.com/l-1amorphousandcrytsallinesolid-240309093040-679d8115/75/L-1-AMORPHOUS-AND-CRYTSALLINE-SOLID-CHAPUT-CHAKMA-9-2048.jpg)

