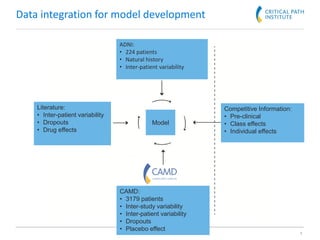
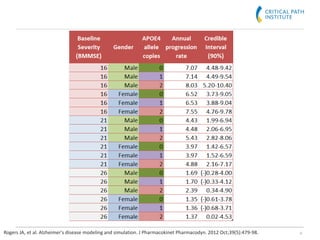
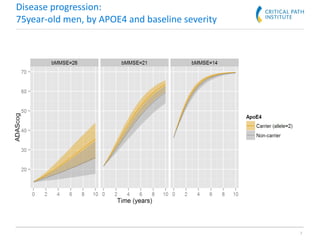
The document discusses a clinical trial simulation tool designed for Alzheimer's disease development, highlighting the reasons for failure in drug development and the importance of trial design and optimization. It outlines the integration of data from various studies and the predictive modeling capabilities to assess disease progression and drug effects, including cognitive assessments and demographic variables. Final conclusions indicate that the tool successfully captures essential clinical trial aspects and received regulatory recognition from the FDA and EMA.