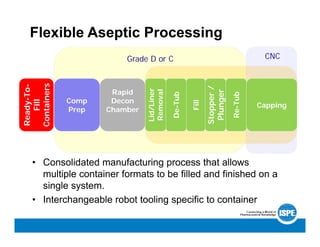
The document compares traditional and flexible aseptic manufacturing, highlighting the benefits of flexible systems in terms of facility space, operational efficiency, and cost-effectiveness. Flexible aseptic processing integrates advanced technologies to enable multiple container formats and reduce routine interventions, while traditional methods rely on dedicated processes. Overall, the shift to flexible aseptic manufacturing streamlines operations, accommodates growing industry demands, and minimizes infrastructure and operational costs.