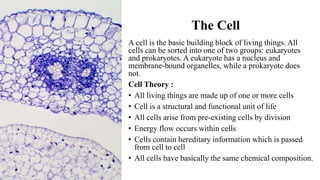
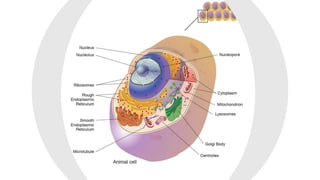
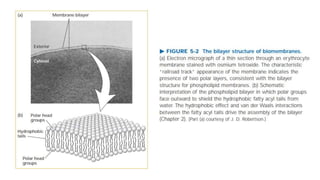
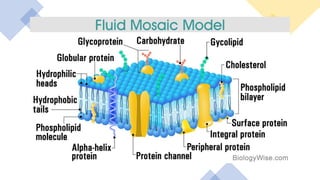
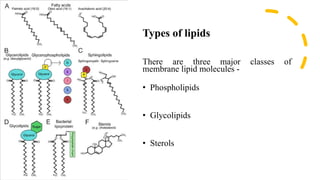
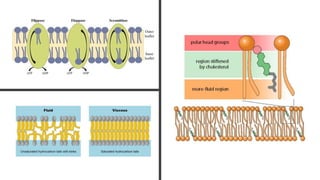
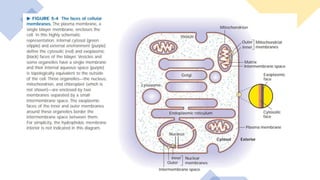
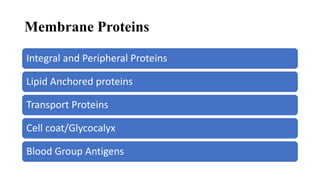
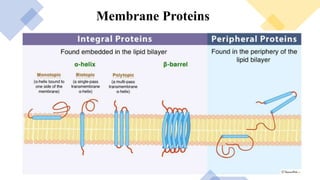
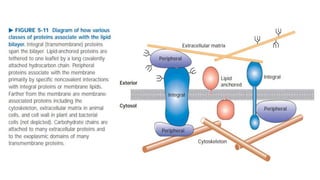
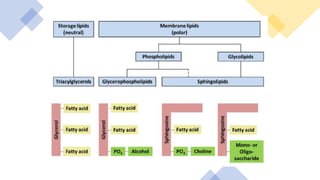
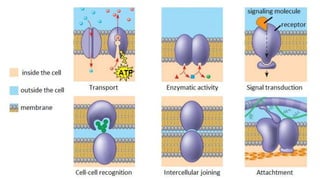
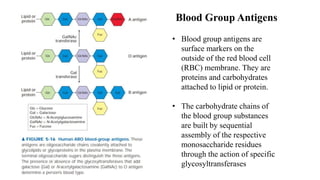
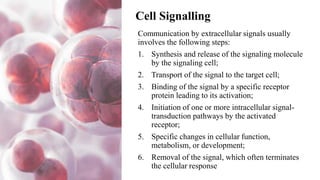
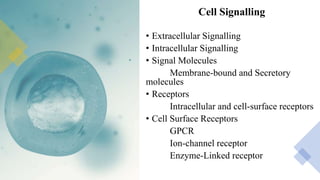
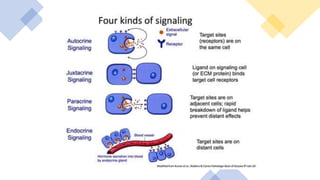
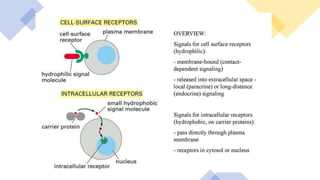
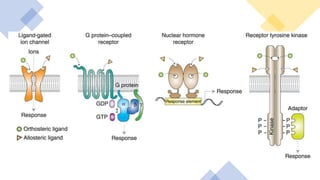
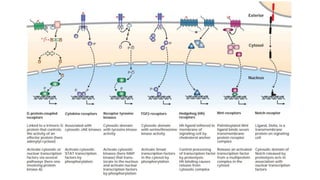
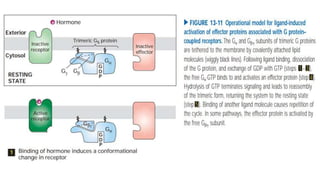
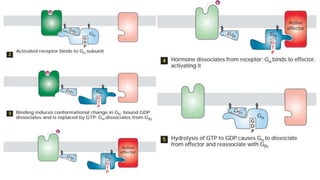
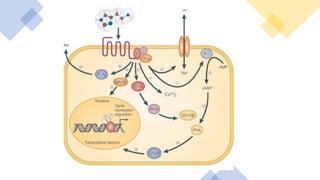
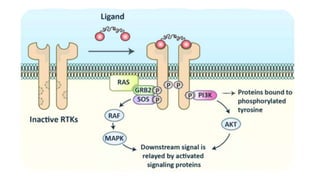
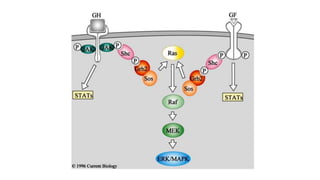
This document discusses why biochemistry is important for physical therapy students. It notes that biochemistry provides an understanding of the physiological processes underlying human movement and function, as biochemistry studies the chemical processes in living organisms. It gives several reasons why biochemistry is key, such as understanding biomolecule structure/function, metabolism, energy production, pharmacology, and molecular bases of disease. The document then provides an overview of basic biochemistry topics like the cell, cell membrane structure and function, types of membrane proteins and lipids, cell signaling pathways, and references for further learning.