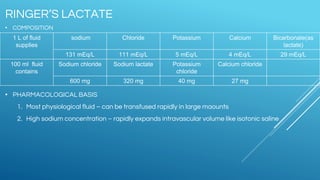
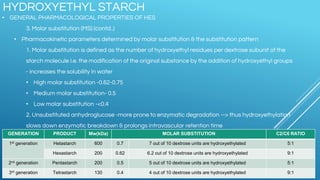
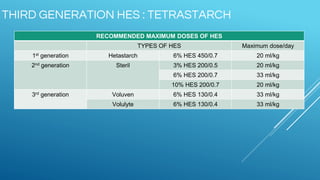
Ringers Lactate and Normal Saline are common crystalloid IV fluids. Ringers Lactate contains electrolytes similar to extracellular fluid and provides bicarbonate which can help correct metabolic acidosis. It is useful for fluid replacement after burns or surgery. Normal Saline contains sodium and chloride in concentrations similar to plasma but has a lower pH and no other electrolytes. It is indicated for hypovolemic shock and conditions requiring sodium replacement. IV fluids can cause complications if given too rapidly, and their properties and indications vary depending on their electrolyte content and concentrations.