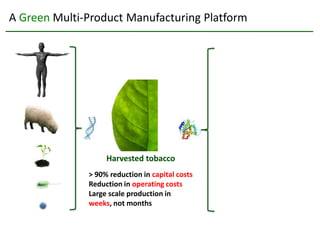
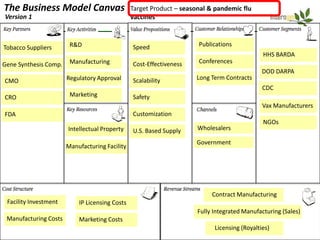
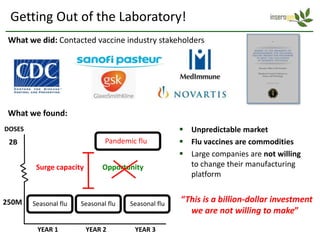
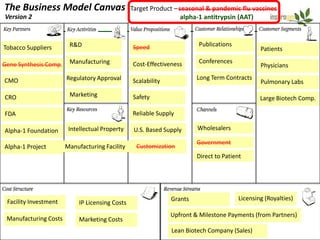
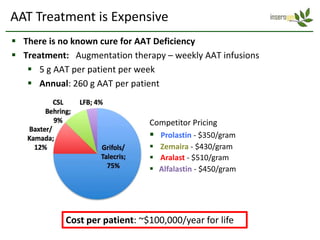
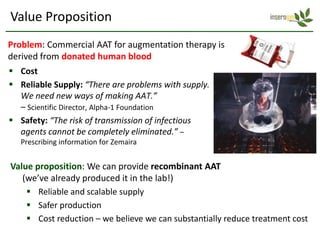
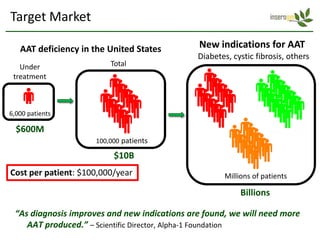
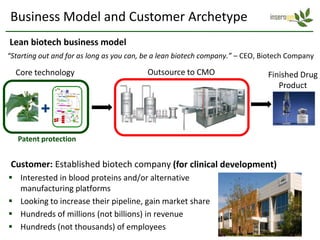
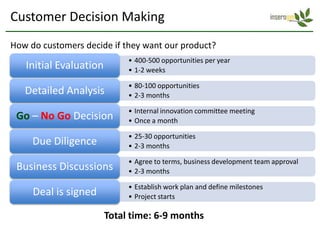
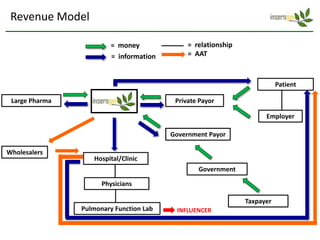
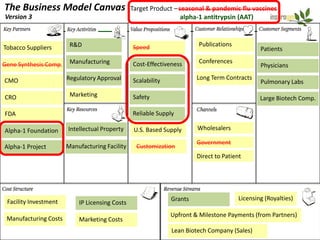
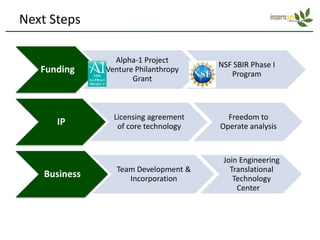
Team Inserogen is developing a technology using tobacco plants as biofactories to produce therapeutics like vaccines and alpha-1 antitrypsin (AAT) more cost effectively and at large scale. Their technology could provide a reliable supply of recombinant AAT at lower cost than current treatments derived from human blood plasma. They are seeking funding and partnerships with biotech companies to advance their technology for producing AAT to treat AAT deficiency, a genetic condition affecting lung health.