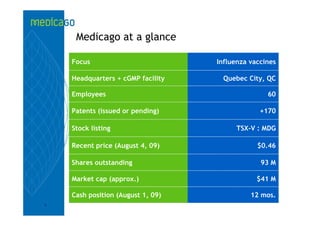
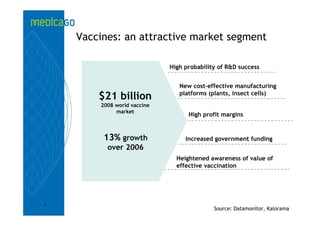
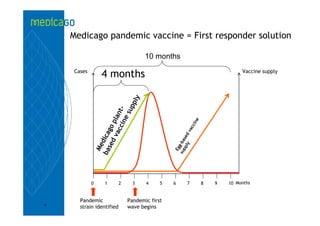
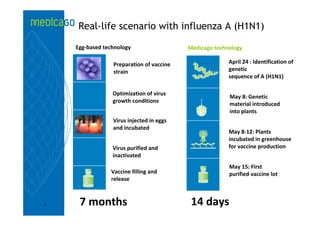
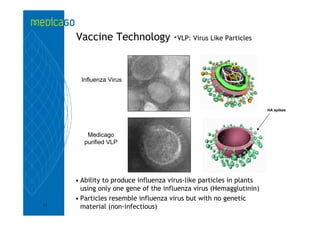
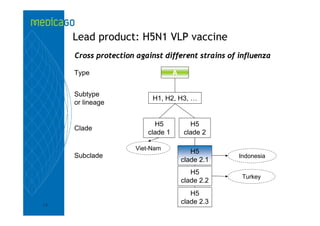
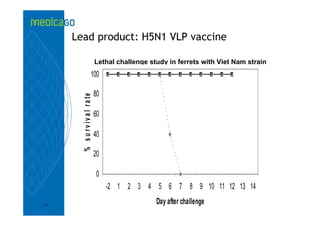
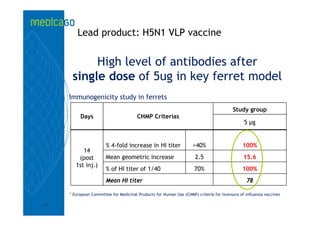
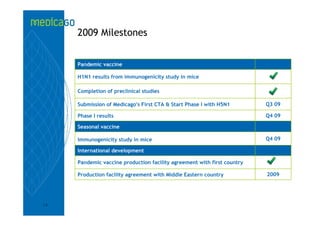
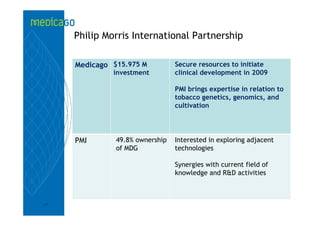
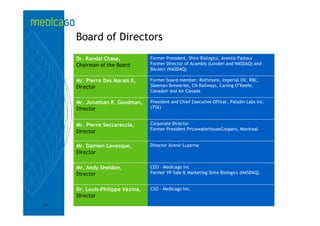
This investor presentation by Medicago provides an overview of their focus on developing influenza vaccines using a plant-based vaccine production platform called Proficia. Their platform uses virus-like particles (VLPs) produced in plants to develop vaccines, which they believe can produce large quantities of pandemic influenza vaccines much faster than egg-based methods. Medicago aims to initiate clinical trials for their pandemic influenza vaccine candidate and leverage those results to accelerate development of a seasonal influenza vaccine, while also exploring VLP opportunities for other diseases beyond influenza.