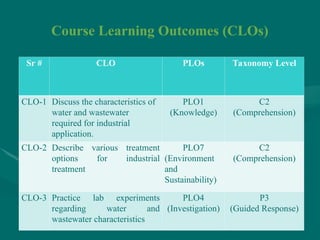
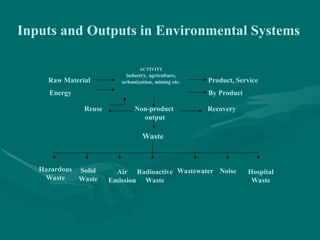
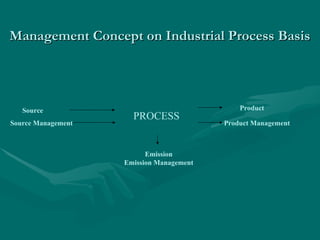
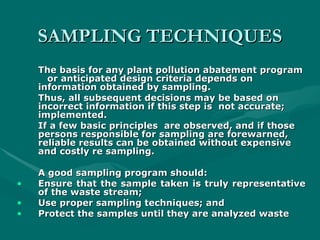
The document addresses industrial pollution and its control, focusing on industrial effluents, types and sources, water treatment techniques, and management of hazardous waste. It elaborates on various pollution types such as air, water, soil, and noise pollution while detailing wastewater characteristics and treatment options across different industries. The text also outlines practical applications and learning outcomes related to the assessment and management of industrial waste.