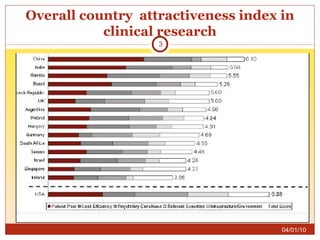
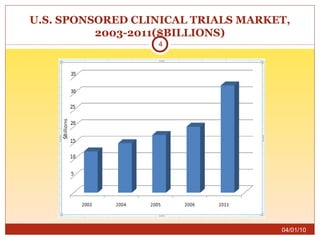
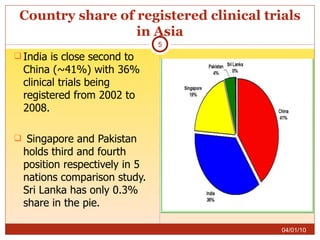
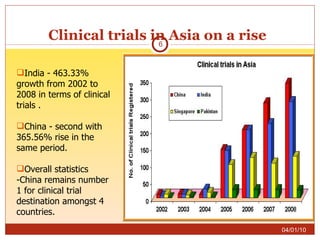
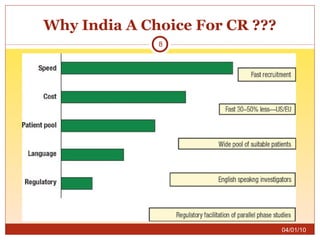
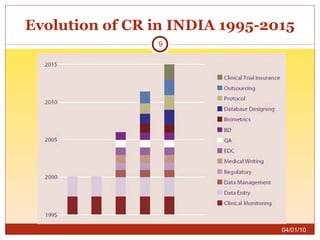
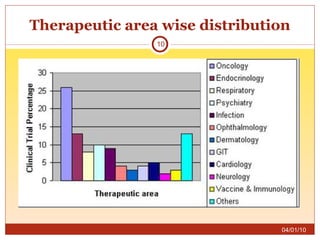
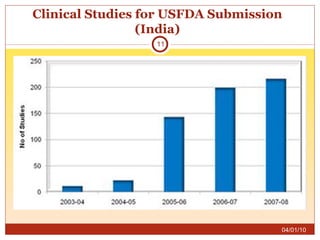
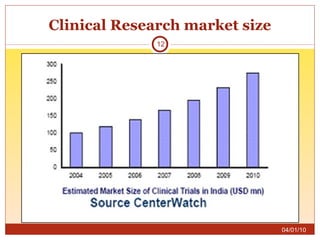
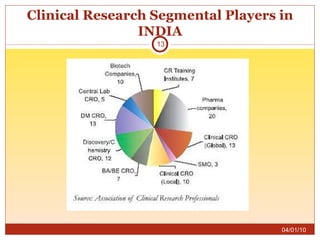
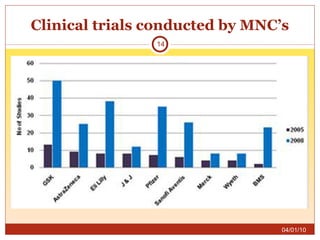
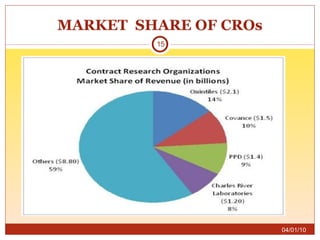
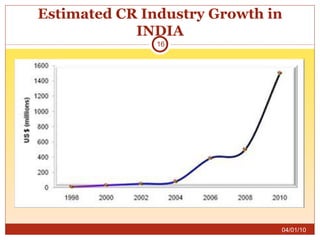
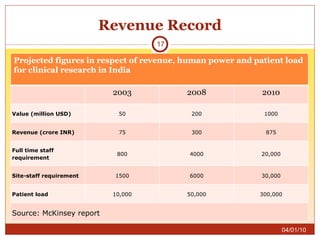
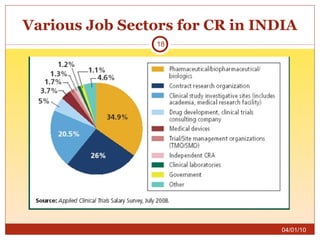
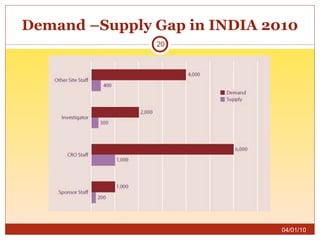
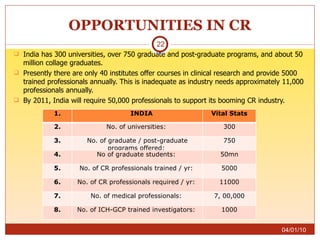
This document summarizes statistics on the clinical research industry. It finds that India has experienced strong growth in clinical trials in recent years and is emerging as a top destination for outsourced clinical research. While the Indian clinical research market is growing rapidly, it remains relatively small compared to the global market and there is a need for more trained professionals to support further industry expansion.