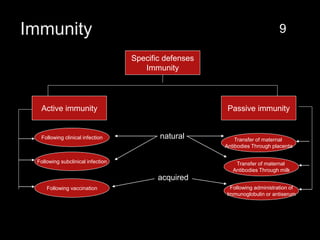
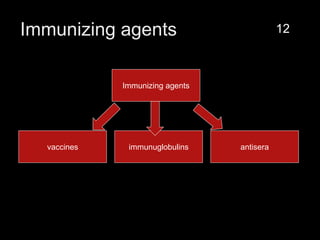
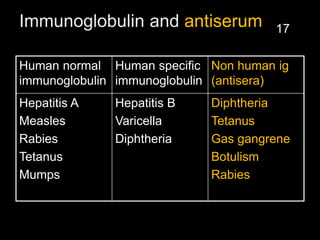
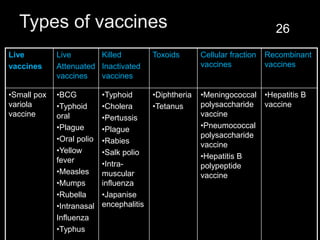
The document provides a comprehensive overview of immunization, detailing its history, definitions, types of vaccines, and their mechanisms of action. It also discusses the importance of vaccines in preventing diseases, the cold chain necessary for vaccine storage, and potential hazards associated with immunization. Additionally, it emphasizes the need for precautions during vaccine administration and outlines recommendations for specific populations such as healthcare workers and pregnant women.