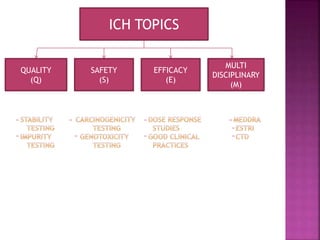
The document summarizes the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH is a joint initiative between regulators and industry to establish and harmonize technical standards for pharmaceutical drug development and regulation. The ICH has guidelines on quality, safety, efficacy, and multidisciplinary topics. Some key achievements and guidelines discussed include standards for stability studies, impurities testing, risk-based pharmaceutical quality management, medical terminology standardization (MedDRA), common technical document format (CTD), and electronic data standards. Dates of finalization are provided for numerous ICH guidelines.