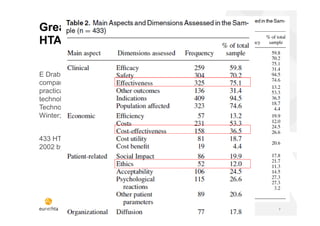
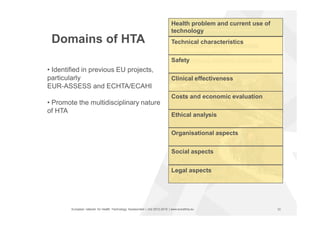
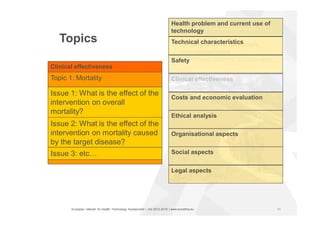
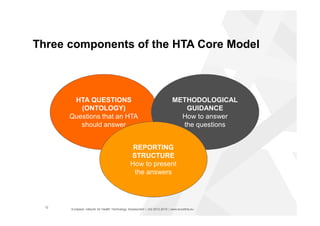
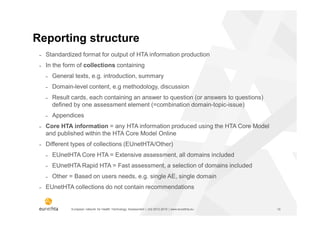
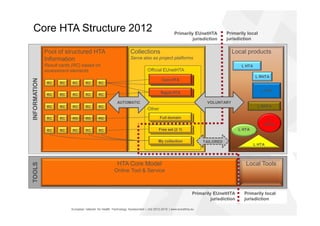
The document discusses the HTA Core Model, which is a framework developed by EUnetHTA to standardize and share health technology assessment information. The key goals of the Core Model are to capture the core elements of HTA that can be shared, enable production of structured HTA information, support joint and local HTA production. The Core Model consists of HTA questions/ontology, methodological guidance, and a reporting structure. It provides a standardized way to organize and report HTA information on a health technology.