How to Get a Medical Device Approved According to New Ukrainian Regulations
•
1 like•14,362 views
1. To get a medical device approved in Ukraine, manufacturers must appoint an authorized representative in Ukraine who can be a distributor or representative office. The manufacturer then identifies the appropriate technical regulations for the device and chooses a conformity assessment procedure. 2. The manufacturer prepares a technical file demonstrating compliance with the requirements in the chosen regulations. They then select a Notified Body to review the file and certify compliance with quality standards. 3. Upon approval, the authorized representative's name and address and the Ukrainian conformity assessment mark must be placed on the device labels and packaging.
Report
Share
Report
Share
Download to read offline
Recommended
Ukraine: What information is mandatory on medical device label? 

The document outlines the mandatory information that must be included on medical device labels for products registered in Ukraine. This includes: 1) the name of the item in Ukrainian, 2) the manufacturer name and address in original language and country of origin in Ukrainian, 3) the registration certificate number and date. Optional information that can be included are main properties, sterility, guarantees, safety warnings, and manufacturer's Ukrainian representative. The label must be in Ukrainian and can include symbols for standards compliance.
Russia: What information is mandatory on medical device label? 

This document summarizes the mandatory information that must be included on medical device labels in Russia. The label must include:
1. The name of the medical device in Russian.
2. Information about the manufacturer including the word "manufacturer" in Russian, the name and address of the manufacturer in the original language, and the country of origin in Russian.
3. The registration number and date of registration for the device in Russia.
4. Information about the device's properties in Russian such as size, sterility, single-use instructions, storage conditions, and shelf life.
202 - Convoluted Hose Datasheet_LR.PDF

This datasheet provides technical information on convoluted hoses. It directs the reader to contact the company for any additional technical details or questions about this or other products. The document appears to be a product datasheet for convoluted hoses issued on September 8, 2030.
Abbreviated 510(k)

Premier Device Consultants was contracted to develop a regulatory strategy for the Laser Therapeutics Company's new medical device called NEULASER, which uses lasers for eye surgery. Premier determined NEULASER is a Class II device that can be cleared through an abbreviated 510(k) by demonstrating substantial equivalence to similar predicate devices. Premier recommended establishing quality management systems, developing documentation for design controls and postmarket surveillance, and preparing all required documents and user fees for the 510(k) submission to obtain FDA clearance to market NEULASER in the United States by the second quarter of 2015.
mock 510(k) for UCSC Extension Regulatory Submissions Devices and Diagnostics...

mock 510(k) for UCSC Extension Regulatory Submissions Devices and Diagnostics...Joanne Pelaschier, RAC, CQA, CQE
The CLARITY Aneurysm Clip is intended for permanent occlusion of cerebral aneurysms. It is made of PEEK polymer, while the predicate Sugita Titanium Aneurysm Clip is made of Elgiloy alloy. Testing showed the CLARITY Clip performs comparable to the predicate in biocompatibility, corrosion resistance, and MRI safety. Though different materials, the CLARITY Clip's intended use, jaw-based occlusion mechanism, and substantial equivalence to the RoG Suture Anchor reference device support that it is as safe and effective as existing aneurysm clips.NDA Paper Final (1)

Streptomirus (Streptovancin) is a new treatment for necrotizing fasciitis (NF) developed by Catalent Pharmaceutical Inc. Non-clinical studies in mice and dogs showed Streptomirus was safer, faster-acting, and more effective than current NF treatments. Clinical trials confirmed Streptomirus' efficacy and safety profile. Catalent is seeking FDA approval to market Streptomirus globally as an orphan drug for NF, an unmet medical need with high mortality. If approved, Streptomirus would provide a more effective treatment option for NF patients.
Regulations related to cardiac stents

a breif description abt the cardiac stents, overview of the cardiac stents, its regulation and marking authorization
Max Hospital Shalimar Bagh Doctor's Prescription

Mr. Manoj Kumar, a 23-year old male, had his platelet count tested on May 29, 2015 at Max Super Speciality Hospital in Shalimar Bagh, New Delhi. His platelet count result was 240 x 109/L, which falls within the reference range of 150.0-410.0 x 109/L for platelet count. The test was ordered by Dr. Vijay Giridhar.
Recommended
Ukraine: What information is mandatory on medical device label? 

The document outlines the mandatory information that must be included on medical device labels for products registered in Ukraine. This includes: 1) the name of the item in Ukrainian, 2) the manufacturer name and address in original language and country of origin in Ukrainian, 3) the registration certificate number and date. Optional information that can be included are main properties, sterility, guarantees, safety warnings, and manufacturer's Ukrainian representative. The label must be in Ukrainian and can include symbols for standards compliance.
Russia: What information is mandatory on medical device label? 

This document summarizes the mandatory information that must be included on medical device labels in Russia. The label must include:
1. The name of the medical device in Russian.
2. Information about the manufacturer including the word "manufacturer" in Russian, the name and address of the manufacturer in the original language, and the country of origin in Russian.
3. The registration number and date of registration for the device in Russia.
4. Information about the device's properties in Russian such as size, sterility, single-use instructions, storage conditions, and shelf life.
202 - Convoluted Hose Datasheet_LR.PDF

This datasheet provides technical information on convoluted hoses. It directs the reader to contact the company for any additional technical details or questions about this or other products. The document appears to be a product datasheet for convoluted hoses issued on September 8, 2030.
Abbreviated 510(k)

Premier Device Consultants was contracted to develop a regulatory strategy for the Laser Therapeutics Company's new medical device called NEULASER, which uses lasers for eye surgery. Premier determined NEULASER is a Class II device that can be cleared through an abbreviated 510(k) by demonstrating substantial equivalence to similar predicate devices. Premier recommended establishing quality management systems, developing documentation for design controls and postmarket surveillance, and preparing all required documents and user fees for the 510(k) submission to obtain FDA clearance to market NEULASER in the United States by the second quarter of 2015.
mock 510(k) for UCSC Extension Regulatory Submissions Devices and Diagnostics...

mock 510(k) for UCSC Extension Regulatory Submissions Devices and Diagnostics...Joanne Pelaschier, RAC, CQA, CQE
The CLARITY Aneurysm Clip is intended for permanent occlusion of cerebral aneurysms. It is made of PEEK polymer, while the predicate Sugita Titanium Aneurysm Clip is made of Elgiloy alloy. Testing showed the CLARITY Clip performs comparable to the predicate in biocompatibility, corrosion resistance, and MRI safety. Though different materials, the CLARITY Clip's intended use, jaw-based occlusion mechanism, and substantial equivalence to the RoG Suture Anchor reference device support that it is as safe and effective as existing aneurysm clips.NDA Paper Final (1)

Streptomirus (Streptovancin) is a new treatment for necrotizing fasciitis (NF) developed by Catalent Pharmaceutical Inc. Non-clinical studies in mice and dogs showed Streptomirus was safer, faster-acting, and more effective than current NF treatments. Clinical trials confirmed Streptomirus' efficacy and safety profile. Catalent is seeking FDA approval to market Streptomirus globally as an orphan drug for NF, an unmet medical need with high mortality. If approved, Streptomirus would provide a more effective treatment option for NF patients.
Regulations related to cardiac stents

a breif description abt the cardiac stents, overview of the cardiac stents, its regulation and marking authorization
Max Hospital Shalimar Bagh Doctor's Prescription

Mr. Manoj Kumar, a 23-year old male, had his platelet count tested on May 29, 2015 at Max Super Speciality Hospital in Shalimar Bagh, New Delhi. His platelet count result was 240 x 109/L, which falls within the reference range of 150.0-410.0 x 109/L for platelet count. The test was ordered by Dr. Vijay Giridhar.
Medical device as per india and usa special reference with 510(k)

1. medical device as per usa:
a) classification
b) 510(k)
c) 510(k) submission process
d) pre market approval(PMA)
2. medical device as per india
a) definition
b) organization of medical device reviewer
c) registration process
d) document required for the registration of medical device as per cdsco
Medical device approval chart for Russia - EMERGO

The regulatory process for medical devices in Russia involves classifying the device, appointing an authorized representative, submitting a registration dossier including technical documentation and clinical data to Roszdravnadzor (RZN), addressing any requests for additional information, and ultimately receiving a registration certificate to market the device in Russia. The process can take 6-12 months depending on the device class.
Procedure for getting the manufacturing license of notified IVDs Products in ...

1) The document outlines the procedure for obtaining a manufacturing license for notified in vitro diagnostic (IVD) products in India. It involves submitting an application with documents to the state and central drug authorities, who may request additional information.
2) If approved, joint inspections are conducted by state and central inspectors. If deficiencies are found, corrections must be made and reinspection may occur. Test batches are produced and evaluated.
3) Upon receiving positive evaluation reports and recommendations, the state authority can issue the manufacturing license, which is valid for 5 years. The process generally takes 6-9 months for notified IVDs. Non-notified IVD licenses have fewer requirements.
Conduction of a remote audit in Ukraine. How is it organized?

MEETING AGENDA:
- When is the remote audit possible and what are the requirements?
- What does the conformity assessment body expect during the audit?
- How to fill in the Checklist for the audit and other documents?
The Medical devices S.R.O.32 (I)/2018

S.R.O.32(I)/2018.— In exercise of the powers conferred by section 23 of the Drug Regulatory Authority of Pakistan Act, 2012 (XXI of 2012), the Drug Regulatory Authority of Pakistan, with the approval of the Federal Government, is pleased to make the following rules, namely
Medical Devices Rules 2017 Implementation

Medical Devices Rules are now enforced for all medical devices. It is important to know about the MDR 2017 and how it affects the Manufacturers, Importers and Distributors of Medical Devices and status of implementation.
CDSCO License.pptx

This document outlines the application process and required documents for various medical device licenses from India's Central Drugs Standard Control Organization (CDSCO). It provides details on:
1. Medical device classification based on risk level into Classes A through D.
2. The application process and documents required for an import license, manufacturing license, loan manufacturing license for Classes A and B devices, and manufacturing license for Classes C and D devices.
3. The documents include regulatory certificates, quality management documentation, device master files, and other technical documents describing the device and quality systems.
5-MHRA(2).pdf

The MHRA has sent a letter to Peter Wei of Lotus Global Co Ltd confirming registration of Xiamen Jiqing Biomedical Technology Co., Ltd. as a manufacturer of in vitro diagnostic medical devices based on the information provided by Wei as their authorized representative. The letter notes that registration does not represent endorsement of the manufacturer's device classifications and that any changes to the registration information must be reported.
medical device regulatory approval in USA

The document discusses the approval process for medical devices in the United States, including an overview of the classification system for medical devices (Class I, II, III), the requirements for each class (e.g. 510(k) notification or premarket approval), and the components required for a 510(k) premarket notification application to the FDA.
Medical devices rules 2015 (summary)

This document outlines the rules and regulations for medical devices in Pakistan, including classification, licensing, registration, labeling, and other requirements. Key points include:
- The Medical Device Board is responsible for regulating medical devices and conformity assessment bodies.
- Medical devices must be classified based on risk and undergo conformity assessment prior to registration and import/sale.
- Manufacturers and importers must obtain establishment licenses, which require meeting quality and premises standards and are valid for 5 years.
- Detailed procedures are defined for licensing, registration, labeling, post-market surveillance, exemptions, and other aspects of the medical device approval process.
Device Registration

The document discusses medical device regulations in India. The Drugs Controller General of India oversees the Central Drugs Standard Control Organisation, which regulates medical devices. Only notified devices are regulated, which include items like syringes, stents, and implants. Device registration requires submitting legal, regulatory, technical, and quality documents and can take 4-5 months. Import licenses for registered devices may take an additional month. The contact details of Accredited Consultants Pvt. Ltd. are provided.
Device Registration in India

The document discusses medical device regulations in India. The Drugs Controller General of India oversees the Central Drugs Standard Control Organisation, which regulates medical devices. Only notified devices are regulated, which include items like syringes, stents, and implants. Device registration requires submitting legal, regulatory, technical, and quality documents and can take 4-5 months. Import licenses for registered devices may take an additional month. The contact details of Accredited Consultants Pvt. Ltd. are provided.
Clinical study approval process in the Russian Federation

This document provides guidance on submitting regulatory approval packages for clinical trials in Russia. It outlines the structure and required contents for initial submission packages to the Ministry of Health of Russia, including protocol, informed consent forms, investigator information, and documentation of Good Manufacturing Practice compliance. It also describes the processes and documentation required for import/export licenses and other submissions such as site additions or patient number increases. The overall purpose is to guide sponsors and CROs in properly assembling regulatory submissions to Russian authorities.
Medical devices: 7 steps to CE-mark, and post-market surveillance

The 7 steps medical device manufacturers have to take to fulfill the requirements of EU-MDR (medical medical device regulation) and to obtain the CE mark. The quick start guide.
RDSO Guidelines for new Vendors- Rajni Ranjan

This document provides guidelines for vendor development and approval for small track machines and tools by the Research Designs and Standards Organisation (RDSO) of the Government of India Ministry of Railways.
It defines key terms, outlines the policy and process for vendor registration and approval, including application assessment, technical capability assessment, prototype testing, and approval procedures. It also covers charges for various activities, validity of approvals, and requirements for new items or extensions. The Standing Committee on Small Track Machines examines cases and makes recommendations to the Railway Board for final approval.
Rdso vendor registration guideline

This document provides guidelines for vendor development and approval for small track machines and tools by the Research Designs and Standards Organisation (RDSO) of the Government of India Ministry of Railways.
It defines key terms, outlines the policy and process for vendor registration and approval, including application assessment, technical capability assessment, prototype testing, and approval procedures. It also covers charges for various activities, validity of approvals, and requirements for new items or extensions. The Standing Committee on Small Track Machines examines cases and makes recommendations to the Railway Board for final approval.
Medical Device Administration In China

This document discusses medical device administration in China. It outlines the competent authorities that regulate medical devices, including the SFDA and Center for Medical Device Evaluation. It also describes the administration activities like registration, production, sales, and post-marketing surveillance. The document then summarizes the laws and regulations regarding medical devices and classifies medical devices into three categories based on risk. It provides details on administration of production, sales, and registration of medical devices in China.
ICH Anu.pptx

This document provides guidance on essential documents that should be maintained for clinical trials. It is divided into three sections based on the stage of the trial: before, during, and after. The document lists important documents such as the investigator's brochure, signed protocol, IRB approval, CRFs, monitoring reports, and financial agreements. It explains that maintaining proper documentation allows evaluation of trial conduct and data quality in compliance with regulations.
Inspection agreement page

This document is a home inspection report for a property located at an address in Avoca, WI that was inspected on 10/21/10. It lists the inspector's name and contact information, as well as details on the purpose, scope, exclusions, limitations, and terms of the inspection. The inspection is intended to detect observable conditions, but cannot cover concealed defects or enter unsafe areas. The report is not intended as a warranty or guarantee, and any disputes would be resolved through arbitration.
China medical device approval chart - EMERGO

1. The document outlines the regulatory process for medical devices in China, including classification of devices and the approval process for Class I, II, and III devices.
2. Class I devices have the simplest approval process, requiring only an administrative review with no submission fees. Class II and III devices require more extensive technical documentation, testing in China, and clinical evaluations.
3. The approval process can take 12-22 months for Class II and III devices and involves appointing a Chinese agent, submitting documentation and application materials in Chinese, testing, and a review by the China Food and Drug Administration before a registration certificate is issued.
Medical Device Labeling in Russia

The document discusses medical device labeling requirements in Russia. It outlines several key Russian laws and regulations pertaining to medical device registration, customs clearance, advertising, sales, distribution, and use. It emphasizes that labeling must be in Russian and match the information in the registration certificate. It recommends using over-labeling with essential information in Russian as the most common way for foreign manufacturers to comply with labeling language requirements. Failure to properly translate labeling could result in seized products or additional taxes and fees.
More Related Content
Similar to How to Get a Medical Device Approved According to New Ukrainian Regulations
Medical device as per india and usa special reference with 510(k)

1. medical device as per usa:
a) classification
b) 510(k)
c) 510(k) submission process
d) pre market approval(PMA)
2. medical device as per india
a) definition
b) organization of medical device reviewer
c) registration process
d) document required for the registration of medical device as per cdsco
Medical device approval chart for Russia - EMERGO

The regulatory process for medical devices in Russia involves classifying the device, appointing an authorized representative, submitting a registration dossier including technical documentation and clinical data to Roszdravnadzor (RZN), addressing any requests for additional information, and ultimately receiving a registration certificate to market the device in Russia. The process can take 6-12 months depending on the device class.
Procedure for getting the manufacturing license of notified IVDs Products in ...

1) The document outlines the procedure for obtaining a manufacturing license for notified in vitro diagnostic (IVD) products in India. It involves submitting an application with documents to the state and central drug authorities, who may request additional information.
2) If approved, joint inspections are conducted by state and central inspectors. If deficiencies are found, corrections must be made and reinspection may occur. Test batches are produced and evaluated.
3) Upon receiving positive evaluation reports and recommendations, the state authority can issue the manufacturing license, which is valid for 5 years. The process generally takes 6-9 months for notified IVDs. Non-notified IVD licenses have fewer requirements.
Conduction of a remote audit in Ukraine. How is it organized?

MEETING AGENDA:
- When is the remote audit possible and what are the requirements?
- What does the conformity assessment body expect during the audit?
- How to fill in the Checklist for the audit and other documents?
The Medical devices S.R.O.32 (I)/2018

S.R.O.32(I)/2018.— In exercise of the powers conferred by section 23 of the Drug Regulatory Authority of Pakistan Act, 2012 (XXI of 2012), the Drug Regulatory Authority of Pakistan, with the approval of the Federal Government, is pleased to make the following rules, namely
Medical Devices Rules 2017 Implementation

Medical Devices Rules are now enforced for all medical devices. It is important to know about the MDR 2017 and how it affects the Manufacturers, Importers and Distributors of Medical Devices and status of implementation.
CDSCO License.pptx

This document outlines the application process and required documents for various medical device licenses from India's Central Drugs Standard Control Organization (CDSCO). It provides details on:
1. Medical device classification based on risk level into Classes A through D.
2. The application process and documents required for an import license, manufacturing license, loan manufacturing license for Classes A and B devices, and manufacturing license for Classes C and D devices.
3. The documents include regulatory certificates, quality management documentation, device master files, and other technical documents describing the device and quality systems.
5-MHRA(2).pdf

The MHRA has sent a letter to Peter Wei of Lotus Global Co Ltd confirming registration of Xiamen Jiqing Biomedical Technology Co., Ltd. as a manufacturer of in vitro diagnostic medical devices based on the information provided by Wei as their authorized representative. The letter notes that registration does not represent endorsement of the manufacturer's device classifications and that any changes to the registration information must be reported.
medical device regulatory approval in USA

The document discusses the approval process for medical devices in the United States, including an overview of the classification system for medical devices (Class I, II, III), the requirements for each class (e.g. 510(k) notification or premarket approval), and the components required for a 510(k) premarket notification application to the FDA.
Medical devices rules 2015 (summary)

This document outlines the rules and regulations for medical devices in Pakistan, including classification, licensing, registration, labeling, and other requirements. Key points include:
- The Medical Device Board is responsible for regulating medical devices and conformity assessment bodies.
- Medical devices must be classified based on risk and undergo conformity assessment prior to registration and import/sale.
- Manufacturers and importers must obtain establishment licenses, which require meeting quality and premises standards and are valid for 5 years.
- Detailed procedures are defined for licensing, registration, labeling, post-market surveillance, exemptions, and other aspects of the medical device approval process.
Device Registration

The document discusses medical device regulations in India. The Drugs Controller General of India oversees the Central Drugs Standard Control Organisation, which regulates medical devices. Only notified devices are regulated, which include items like syringes, stents, and implants. Device registration requires submitting legal, regulatory, technical, and quality documents and can take 4-5 months. Import licenses for registered devices may take an additional month. The contact details of Accredited Consultants Pvt. Ltd. are provided.
Device Registration in India

The document discusses medical device regulations in India. The Drugs Controller General of India oversees the Central Drugs Standard Control Organisation, which regulates medical devices. Only notified devices are regulated, which include items like syringes, stents, and implants. Device registration requires submitting legal, regulatory, technical, and quality documents and can take 4-5 months. Import licenses for registered devices may take an additional month. The contact details of Accredited Consultants Pvt. Ltd. are provided.
Clinical study approval process in the Russian Federation

This document provides guidance on submitting regulatory approval packages for clinical trials in Russia. It outlines the structure and required contents for initial submission packages to the Ministry of Health of Russia, including protocol, informed consent forms, investigator information, and documentation of Good Manufacturing Practice compliance. It also describes the processes and documentation required for import/export licenses and other submissions such as site additions or patient number increases. The overall purpose is to guide sponsors and CROs in properly assembling regulatory submissions to Russian authorities.
Medical devices: 7 steps to CE-mark, and post-market surveillance

The 7 steps medical device manufacturers have to take to fulfill the requirements of EU-MDR (medical medical device regulation) and to obtain the CE mark. The quick start guide.
RDSO Guidelines for new Vendors- Rajni Ranjan

This document provides guidelines for vendor development and approval for small track machines and tools by the Research Designs and Standards Organisation (RDSO) of the Government of India Ministry of Railways.
It defines key terms, outlines the policy and process for vendor registration and approval, including application assessment, technical capability assessment, prototype testing, and approval procedures. It also covers charges for various activities, validity of approvals, and requirements for new items or extensions. The Standing Committee on Small Track Machines examines cases and makes recommendations to the Railway Board for final approval.
Rdso vendor registration guideline

This document provides guidelines for vendor development and approval for small track machines and tools by the Research Designs and Standards Organisation (RDSO) of the Government of India Ministry of Railways.
It defines key terms, outlines the policy and process for vendor registration and approval, including application assessment, technical capability assessment, prototype testing, and approval procedures. It also covers charges for various activities, validity of approvals, and requirements for new items or extensions. The Standing Committee on Small Track Machines examines cases and makes recommendations to the Railway Board for final approval.
Medical Device Administration In China

This document discusses medical device administration in China. It outlines the competent authorities that regulate medical devices, including the SFDA and Center for Medical Device Evaluation. It also describes the administration activities like registration, production, sales, and post-marketing surveillance. The document then summarizes the laws and regulations regarding medical devices and classifies medical devices into three categories based on risk. It provides details on administration of production, sales, and registration of medical devices in China.
ICH Anu.pptx

This document provides guidance on essential documents that should be maintained for clinical trials. It is divided into three sections based on the stage of the trial: before, during, and after. The document lists important documents such as the investigator's brochure, signed protocol, IRB approval, CRFs, monitoring reports, and financial agreements. It explains that maintaining proper documentation allows evaluation of trial conduct and data quality in compliance with regulations.
Inspection agreement page

This document is a home inspection report for a property located at an address in Avoca, WI that was inspected on 10/21/10. It lists the inspector's name and contact information, as well as details on the purpose, scope, exclusions, limitations, and terms of the inspection. The inspection is intended to detect observable conditions, but cannot cover concealed defects or enter unsafe areas. The report is not intended as a warranty or guarantee, and any disputes would be resolved through arbitration.
China medical device approval chart - EMERGO

1. The document outlines the regulatory process for medical devices in China, including classification of devices and the approval process for Class I, II, and III devices.
2. Class I devices have the simplest approval process, requiring only an administrative review with no submission fees. Class II and III devices require more extensive technical documentation, testing in China, and clinical evaluations.
3. The approval process can take 12-22 months for Class II and III devices and involves appointing a Chinese agent, submitting documentation and application materials in Chinese, testing, and a review by the China Food and Drug Administration before a registration certificate is issued.
Similar to How to Get a Medical Device Approved According to New Ukrainian Regulations (20)
Medical device as per india and usa special reference with 510(k)

Medical device as per india and usa special reference with 510(k)
Procedure for getting the manufacturing license of notified IVDs Products in ...

Procedure for getting the manufacturing license of notified IVDs Products in ...
Conduction of a remote audit in Ukraine. How is it organized?

Conduction of a remote audit in Ukraine. How is it organized?
Clinical study approval process in the Russian Federation

Clinical study approval process in the Russian Federation
Medical devices: 7 steps to CE-mark, and post-market surveillance

Medical devices: 7 steps to CE-mark, and post-market surveillance
More from Alexey Stepanov
Medical Device Labeling in Russia

The document discusses medical device labeling requirements in Russia. It outlines several key Russian laws and regulations pertaining to medical device registration, customs clearance, advertising, sales, distribution, and use. It emphasizes that labeling must be in Russian and match the information in the registration certificate. It recommends using over-labeling with essential information in Russian as the most common way for foreign manufacturers to comply with labeling language requirements. Failure to properly translate labeling could result in seized products or additional taxes and fees.
Eurasian integration alexey stepanov-informa v20150619

The document discusses upcoming harmonization of medical device regulations within the Eurasian Economic Union (EEU) between Russia, Belarus, and Kazakhstan. Key points include:
- A common medical device market within the EEU is expected to launch on January 1, 2016 under new harmonized rules.
- Many regulations still need to be adopted by the end of 2015 to provide guidance for implementation.
- Transition periods and potential delays mean full harmonization may not be achieved immediately.
- Manufacturers will need to reallocate resources to adjust to the new regulatory system over time, including re-registering products under the new rules by 2022.
Changes and Modifications

This document summarizes regulations for changes and modifications to registered medical devices across Russia, Kazakhstan, and Belarus. It outlines three procedures - amendment, notification, and re-registration. An amendment procedure allows for name, address, or accessory changes and is fastest in Russia and Kazakhstan but requires full re-registration in Belarus. A notification procedure in Russia addresses certain indication, storage, marking, or software changes and is approved within 30 days. All other changes generally require the full re-registration process.
Kazakhstan: What information is mandatory on medical device label?

Kazakhstan: What information is mandatory on medical device label?
5 questions on safety reporting for medical devices in CIS region

This document summarizes safety reporting requirements for medical devices in CIS countries, including Russia, Belarus, Ukraine, and Kazakhstan. It outlines what information must be reported, the regulations requiring reporting, reporting deadlines, and how and where to report. The key information that must be reported includes adverse reactions, undesirable events during usage, circumstances endangering patient or health professional life or health, and device interactions. Regulations and reporting bodies differ by country but generally require reporting within 10-20 days to the relevant health ministry or inspectorate.
Localization 2014

The Russian Ministry of Industry and Trade reconsidered restrictions on the supply of medical devices for state procurement in July 2014. The restrictions affect state and municipal procurements of medical devices from a closed list. If at least two bids with local products from Russia, Belarus or Kazakhstan are submitted for tender, buyers will be unable to procure medical devices originating from other countries. The local products must be confirmed by a certificate of origin issued by a local authorized agency. Certain categories of medical devices are subject to the restrictions, including syringes, osteosynthesis implants, ECG devices, and surgical instruments.
How to provide proper photos for medical device registration dossier? 

The document provides guidelines for submitting photo documentation of medical devices for registration purposes, requiring at least one high resolution photo sized 18x24cm showing the device and all accessories, with each item clearly identified either through graphic labeling or direct labeling on the photo. For similar devices of different sizes, one photo is acceptable, and for dossiers covering multiple different devices, one photo per type is sufficient along with a product catalog.
Let us put some clarity on VAT topic

This document outlines the value-added tax (VAT) rates for medical devices in Russia both past and present. It states that historically, medical equipment was subject to a 0% VAT rate, medical products had an 18% VAT rate, and the exact lists of qualifying items were defined in government orders from 2002 and 2008. As of January 1, 2013, a new law unified these terms as "medical devices" and introduced some VAT rate changes, making the VAT rules less clear. The document concludes that additional low-level legislative changes on medical device VAT rates in Russia may still be forthcoming.
15 Steps to Register a Medical Device in Russia

The document outlines 15 steps to register a medical device in Russia, including contracting with a testing laboratory, importing samples, conducting toxicological and technical testing, submitting dossier for evaluation, receiving expert reviews and approvals, conducting clinical trials, and obtaining final registration approval. It provides estimated timelines for each step ranging from 1 to 30 days, noting these do not reflect current delays and that resolving issues with the dossier or additional regulatory requests can take up to 30 more days.
How Can You Group Medical Devices for Registration in Russia? 

Slide explains the principles of product grouping for submission for registration of medical device in RosZdravNadzor (Russia).
More from Alexey Stepanov (12)
Eurasian integration alexey stepanov-informa v20150619

Eurasian integration alexey stepanov-informa v20150619
Kazakhstan: What information is mandatory on medical device label?

Kazakhstan: What information is mandatory on medical device label?
5 questions on safety reporting for medical devices in CIS region

5 questions on safety reporting for medical devices in CIS region
How to provide proper photos for medical device registration dossier? 

How to provide proper photos for medical device registration dossier?
How Can You Group Medical Devices for Registration in Russia? 

How Can You Group Medical Devices for Registration in Russia?
Recently uploaded
TEST BANK FOR Health Assessment in Nursing 7th Edition by Weber Chapters 1 - ...

TEST BANK FOR Health Assessment in Nursing 7th Edition by Weber Chapters 1 - ...rightmanforbloodline
TEST BANK FOR Health Assessment in Nursing 7th Edition by Weber Chapters 1 - 34.
TEST BANK FOR Health Assessment in Nursing 7th Edition by Weber Chapters 1 - 34.
TEST BANK FOR Health Assessment in Nursing 7th Edition by Weber Chapters 1 - 34.2024 Media Preferences of Older Adults: Consumer Survey and Marketing Implica...

When it comes to creating marketing strategies that target older adults, it is crucial to have insight into their media habits and preferences. Understanding how older adults consume and use media is key to creating acquisition and retention strategies. We recently conducted our seventh annual survey to gain insight into the media preferences of older adults in 2024. Here are the survey responses and marketing implications that stood out to us.
Columbia毕业证书退学办理

哥伦比亚大学毕业证成绩单(留信网认证)【176555708微信】24小时办理学位证书编号怎么查
Columbia文凭毕业证学位证(176555708微信)加急制作哥伦比亚大学毕业证认证成绩单密封邮寄|Columbia研究生毕业证书学位证书|哥伦比亚大学Office Transcript美国I20修改|Columbia Office Transcript diploma 学历认证
Columbia毕业证【微信176555708】哥伦比亚大学毕业证书原版↑制作哥伦比亚大学学历认证文凭办理哥伦比亚大学留信网认证,留学回国办理毕业证成绩单文凭学历认证【微信176555708】专业为海外学子办理毕业证成绩单、文凭制作,学历仿制,回国人员证明、做文凭,研究生、本科、硕士学历认证、留信认证、结业证、学位证书样本、美国教育部认证百分百真实存档可查】
Faridkot ℂ𝕒𝕝𝕝 𝔾𝕚𝕣𝕝𝕤 7742996321 ℂ𝕒𝕝𝕝 𝔾𝕚𝕣𝕝𝕤 Faridkot

Faridkot ℂ𝕒𝕝𝕝 𝔾𝕚𝕣𝕝𝕤 7742996321 ℂ𝕒𝕝𝕝 𝔾𝕚𝕣𝕝𝕤 Faridkot
Fit to Fly PCR Covid Testing at our Clinic Near You

A Fit-to-Fly PCR Test is a crucial service for travelers needing to meet the entry requirements of various countries or airlines. This test involves a polymerase chain reaction (PCR) test for COVID-19, which is considered the gold standard for detecting active infections. At our travel clinic in Leeds, we offer fast and reliable Fit to Fly PCR testing, providing you with an official certificate verifying your negative COVID-19 status. Our process is designed for convenience and accuracy, with quick turnaround times to ensure you receive your results and certificate in time for your departure. Trust our professional and experienced medical team to help you travel safely and compliantly, giving you peace of mind for your journey.www.nxhealthcare.co.uk
Psychedelic Retreat Portugal - Escape to Lighthouse Retreats for an unforgett...

Our aim is to organise conscious gatherings and retreats for open and inquisitive minds and souls, with and without the assistance of sacred plants.
Mental Health and Physical Wellbeing.pdf

Mental Health and well-being Presentation. Exploring innovative approaches and strategies for enhancing mental well-being. Discover cutting-edge research, effective strategies, and practical methods for fostering mental well-being.
VEDANTA AIR AMBULANCE SERVICES IN REWA AT A COST-EFFECTIVE PRICE.pdf

Air Ambulance Services In Rewa works in close coordination with ground-based emergency services, including local Emergency Medical Services, fire departments, and law enforcement agencies.
More@: https://tinyurl.com/2shrryhx
More@: https://tinyurl.com/5n8h3wp8
Enhancing Hip and Knee Arthroplasty Precision with Preoperative CT and MRI Im...

Enhancing Hip and Knee Arthroplasty Precision with Preoperative CT and MRI Im...Pristyn Care Reviews
Precision becomes a byword, most especially in such procedures as hip and knee arthroplasty. The success of these surgeries is not just dependent on the skill and experience of the surgeons but is extremely dependent on preoperative planning. Recognizing this important need, Pristyn Care commits itself to the integration of advanced imaging technologies like CT (Computed Tomography) and MRI (Magnetic Resonance Imaging) into the surgical planning process.The Importance of Black Women Understanding the Chemicals in Their Personal C...

Certain chemicals, such as phthalates and parabens, can disrupt the body's hormones and have significant effects on health. According to data, hormone-related health issues such as uterine fibroids, infertility, early puberty and more aggressive forms of breast and endometrial cancers disproportionately affect Black women. Our guest speaker, Jasmine A. McDonald, PhD, an Assistant Professor in the Department of Epidemiology at Columbia University in New York City, discusses the scientific reasons why Black women should pay attention to specific chemicals in their personal care products, like hair care, and ways to minimize their exposure.
Hypotension and role of physiotherapy in it

This particular slides consist of- what is hypotension,what are it's causes and it's effect on body, risk factors, symptoms,complications, diagnosis and role of physiotherapy in it.
This slide is very helpful for physiotherapy students and also for other medical and healthcare students.
Here is the summary of hypotension:
Hypotension, or low blood pressure, is when the pressure of blood circulating in the body is lower than normal or expected. It's only a problem if it negatively impacts the body and causes symptoms. Normal blood pressure is usually between 90/60 mmHg and 120/80 mmHg, but pressures below 90/60 are generally considered hypotensive.
Monopoly PCD Pharma Franchise in Tripura

Our company incorporates various drug formulations covering pharma tablets, syrups, capsules, gels, sachets, ointments, creams, injectables.
Solution manual for managerial accounting 18th edition by ray garrison eric n...

Solution manual for managerial accounting 18th edition by ray garrison eric n...rightmanforbloodline
Solution manual for managerial accounting 18th edition by ray garrison eric noreen and peter brewer_compressed
Solution manual for managerial accounting 18th edition by ray garrison eric noreen and peter brewer_compressedThe Ultimate Guide in Setting Up Market Research System in Health-Tech

How to effectively start market research in the health tech industry by defining objectives, crafting problem statements, selecting methods, identifying data collection sources, and setting clear timelines. This guide covers all the preliminary steps needed to lay a strong foundation for your research.
"Market Research it too text-booky, I am in the market for a decade, I am living research book" this is what the founder I met on the event claimed, few of my colleagues rolled their eyes. Its true that one cannot over look the real life experience, but one cannot out beat structured gold mine of market research.
Many 0 to 1 startup founders often overlook market research, but this critical step can make or break a venture, especially in health tech.
But Why do they skip it?
Limited resources—time, money, and manpower—are common culprits.
"In fact, a survey by CB Insights found that 42% of startups fail due to no market need, which is like building a spaceship to Mars only to realise you forgot the fuel."
Sudharsan Srinivasan
Operational Partner Pitchworks VC Studio
Overconfidence in their product’s success leads founders to assume it will naturally find its market, especially in health tech where patient needs, entire system issues and regulatory requirements are as complex as trying to perform brain surgery with a butter knife. Additionally, the pressure to launch quickly and the belief in their own intuition further contribute to this oversight. Yet, thorough market research in health tech could be the key to transforming a startup's vision into a life-saving reality, instead of a medical mishap waiting to happen.
Example of Market Research working
Innovaccer, founded by Abhinav Shashank in 2014, focuses on improving healthcare delivery through data-driven insights and interoperability solutions. Before launching their platform, Innovaccer conducted extensive market research to understand the challenges faced by healthcare organizations and the potential for innovation in healthcare IT.
Identifying Pain Points: Innovaccer surveyed healthcare providers to understand their difficulties with data integration, care coordination, and patient engagement. They found widespread frustration with siloed systems and inefficient workflows.
Competitive Analysis: Analyzed competitors offering similar solutions in healthcare analytics and interoperability. Identified gaps in comprehensive data aggregation, real-time analytics, and actionable insights.
Regulatory Compliance: Ensured their platform complied with HIPAA and other healthcare data privacy regulations. This compliance was crucial to gaining trust from healthcare providers wary of data security issues.
Customer Validation: Conducted pilot programs with several healthcare organizations to validate the platform's effectiveness in improving care outcomes and operational efficiency. Gathered feedback to refine features and user interface.
nurs fpx 4050 assessment 4 final care coordination plan.pdf

nurs fpx 4050 assessment 4 final care coordination plan.pdf
Know Latest Hiranandani Hospital Powai News.pdf

As Mumbai's premier kidney transplant and donation center, L H Hiranandani Hospital Powai is not just a medical facility; it's a beacon of hope where cutting-edge science meets compassionate care, transforming lives and redefining the standards of kidney health in India.
R3 Stem Cell Therapy: A New Hope for Women with Ovarian Failure

Discover the groundbreaking advancements in stem cell therapy by R3 Stem Cell, offering new hope for women with ovarian failure. This innovative treatment aims to restore ovarian function, improve fertility, and enhance overall well-being, revolutionizing reproductive health for women worldwide.
Hyderabad Call Girls 7023059433 High Profile Escorts Service Hyderabad

Hyderabad Call Girls 7023059433 High Profile Escorts Service Hyderabad
National Rural Health Mission(NRHM).pptx

This document describes the NRHM Scheme, its goals, objectives, components, and new initiatives.
Recently uploaded (20)
TEST BANK FOR Health Assessment in Nursing 7th Edition by Weber Chapters 1 - ...

TEST BANK FOR Health Assessment in Nursing 7th Edition by Weber Chapters 1 - ...
2024 Media Preferences of Older Adults: Consumer Survey and Marketing Implica...

2024 Media Preferences of Older Adults: Consumer Survey and Marketing Implica...
Faridkot ℂ𝕒𝕝𝕝 𝔾𝕚𝕣𝕝𝕤 7742996321 ℂ𝕒𝕝𝕝 𝔾𝕚𝕣𝕝𝕤 Faridkot

Faridkot ℂ𝕒𝕝𝕝 𝔾𝕚𝕣𝕝𝕤 7742996321 ℂ𝕒𝕝𝕝 𝔾𝕚𝕣𝕝𝕤 Faridkot
Fit to Fly PCR Covid Testing at our Clinic Near You

Fit to Fly PCR Covid Testing at our Clinic Near You
Psychedelic Retreat Portugal - Escape to Lighthouse Retreats for an unforgett...

Psychedelic Retreat Portugal - Escape to Lighthouse Retreats for an unforgett...
VEDANTA AIR AMBULANCE SERVICES IN REWA AT A COST-EFFECTIVE PRICE.pdf

VEDANTA AIR AMBULANCE SERVICES IN REWA AT A COST-EFFECTIVE PRICE.pdf
Enhancing Hip and Knee Arthroplasty Precision with Preoperative CT and MRI Im...

Enhancing Hip and Knee Arthroplasty Precision with Preoperative CT and MRI Im...
The Importance of Black Women Understanding the Chemicals in Their Personal C...

The Importance of Black Women Understanding the Chemicals in Their Personal C...
Solution manual for managerial accounting 18th edition by ray garrison eric n...

Solution manual for managerial accounting 18th edition by ray garrison eric n...
The Ultimate Guide in Setting Up Market Research System in Health-Tech

The Ultimate Guide in Setting Up Market Research System in Health-Tech
nurs fpx 4050 assessment 4 final care coordination plan.pdf

nurs fpx 4050 assessment 4 final care coordination plan.pdf
R3 Stem Cell Therapy: A New Hope for Women with Ovarian Failure

R3 Stem Cell Therapy: A New Hope for Women with Ovarian Failure
Hyderabad Call Girls 7023059433 High Profile Escorts Service Hyderabad

Hyderabad Call Girls 7023059433 High Profile Escorts Service Hyderabad
How to Get a Medical Device Approved According to New Ukrainian Regulations
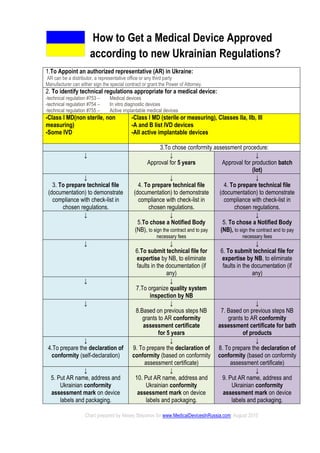
- 1. How to Get a Medical Device Approved according to new Ukrainian Regulations? 1.To Appoint an authorized representative (AR) in Ukraine: AR can be a distributor, a representative office or any third party Manufacturer can either sign the special contract or grant the Power of Attorney. 2. To identify technical regulations appropriate for a medical device: -technical regulation #753 – Medical devices -technical regulation #754 – In vitro diagnostic devices -technical regulation #755 – Active implantable medical devices -Class I MD(non sterile, non measuring) -Some IVD -Class I MD (sterile or measuring), Classes IIa, IIb, III -A and B list IVD devices -All active implantable devices 3.To chose conformity assessment procedure: ↓ ↓ Approval for 5 years ↓ Approval for production batch (lot) ↓ 3. To prepare technical file (documentation) to demonstrate compliance with check-list in chosen regulations. ↓ 4. To prepare technical file (documentation) to demonstrate compliance with check-list in chosen regulations. ↓ 4. To prepare technical file (documentation) to demonstrate compliance with check-list in chosen regulations. ↓ ↓ 5.To chose a Notified Body (NB), to sign the contract and to pay necessary fees ↓ 5. To chose a Notified Body (NB), to sign the contract and to pay necessary fees ↓ ↓ 6.To submit technical file for expertise by NB, to eliminate faults in the documentation (if any) ↓ 6. To submit technical file for expertise by NB, to eliminate faults in the documentation (if any) ↓ ↓ 7.To organize quality system inspection by NB ↓ ↓ 8.Based on previous steps NB grants to AR conformity assessment certificate for 5 years ↓ 7. Based on previous steps NB grants to AR conformity assessment certificate for bath of products ↓ 4.To prepare the declaration of conformity (self-declaration) ↓ 9. To prepare the declaration of conformity (based on conformity assessment certificate) ↓ 8. To prepare the declaration of conformity (based on conformity assessment certificate) ↓ 5. Put AR name, address and Ukrainian conformity assessment mark on device labels and packaging. ↓ 10. Put AR name, address and Ukrainian conformity assessment mark on device labels and packaging. ↓ 9. Put AR name, address and Ukrainian conformity assessment mark on device labels and packaging. Chart prepared by Alexey Stepanov for www.MedicalDevicesInRussia.com, August 2015
