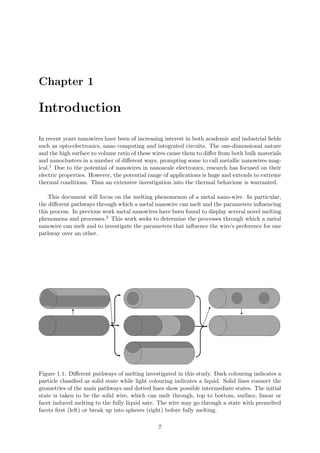
This document summarizes the different pathways a cylindrical metal nanowire can melt through: surface melting, linear melting, and facet induced melting. A simple model is developed based on free energy considerations to predict the preferred melting pathway based on the wire radius and interfacial energy differences. Molecular dynamics simulations of aluminum nanowires demonstrate the different pathways and confirm radius is an important parameter. The simulations also show the interface energies and shrinkage of non-melting facets influence the melting pathway.