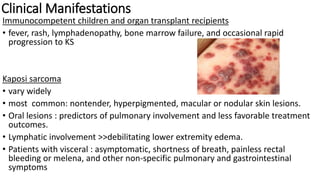
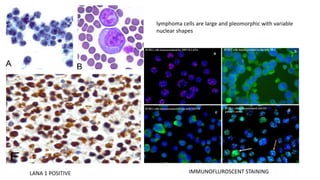
Human herpesvirus-8 (HHV-8), associated with Kaposi sarcoma (KS), has varying seroprevalence rates based on geography and risk groups, particularly among men who have sex with men and individuals with HIV. Clinical manifestations of HHV-8 infections can include KS, primary effusion lymphoma, and multicentric Castleman’s disease, with diverse symptoms affecting different patients. Diagnosis requires tissue examination, while treatment typically involves chemotherapy combined with antiretroviral therapy, with strategies differing depending on the specific manifestations and disease progression.


![Risk population
• men who have sex with men (MSM)
• persons with HIV infection
• MSW without HIV sero prevalence ranges from 13% to 20%
• MSW with HIV sero prevalence ranges from 30% to 35%
• Injection drug
• CD4 T lymphocyte [CD4] cell counts <200 cells/mm3](https://image.slidesharecdn.com/hhv8-230221175540-3ad4f4d3/85/HHV-8-pptx-4-320.jpg)
![HHV 8 a/w
• Kaposi sarcoma (KS) including classic, endemic, transplant-related,
and AIDS-related,
• primary effusion lymphoma [PEL] and solid organ variants
• multicentric Castleman’s disease (MCD)](https://image.slidesharecdn.com/hhv8-230221175540-3ad4f4d3/85/HHV-8-pptx-5-320.jpg)