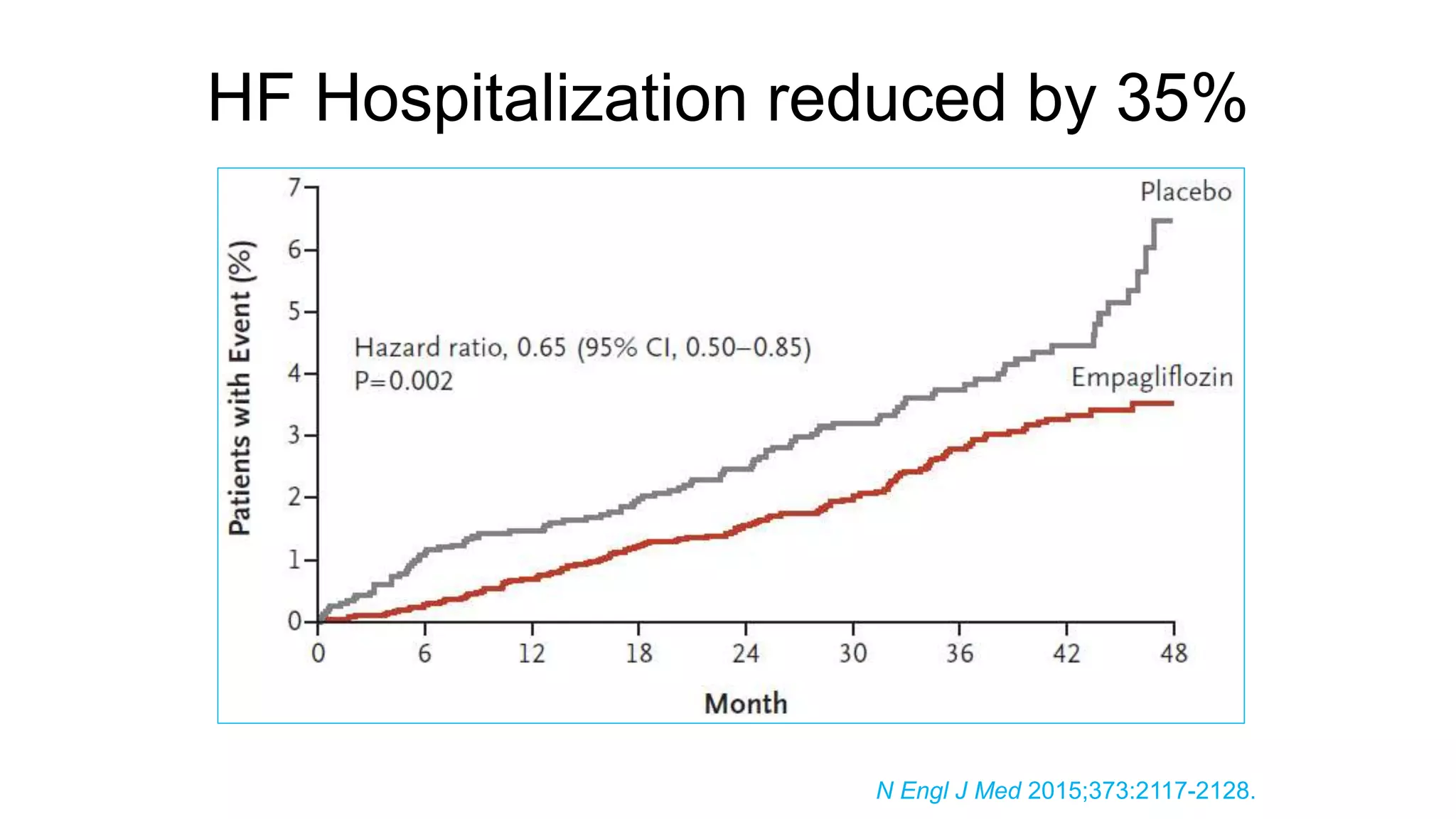
The document discusses the management of type 2 diabetes mellitus (T2DM) and outlines various therapeutic options, emphasizing the importance of individualized treatment plans based on patient characteristics and preferences. It highlights the roles of different medications, potential side effects, and guidelines from organizations like ADA and AACE regarding glycemic targets and combination therapies. Additionally, it provides insights into ongoing research and evolving standards of care for T2DM management.














![2012 ADA-EASD ‘Position Statement’: Management of
Hyperglycemia in T2DM: A Patient-Centered Approach
GLUCOSE-LOWERING THERAPY
• Glycemic targets
- HbA1c < 7.0% (mean PG 150-160 mg/dl [8.3-8.9 mmol/l])
- Pre-prandial PG <130 mg/dl (7.2 mmol/l)
- Post-prandial PG <180 mg/dl (10.0 mmol/l)
- Individualization is key:
Tighter targets (6.0 - 6.5%) - younger, healthier
Looser targets (7.5 - 8.0%+) - older, comorbidities,
hypoglycemia prone, etc.
PG = plasma glucose Diabetes Care 2012;35:1364–1379
Diabetologia 2012;55:1577–1596
• Pharmacological options
- Individualize drug choice
- Minimize adverse effects, especially hypoglycemia
- Patient-centered care](https://image.slidesharecdn.com/j03dmguidelines-171003141921/75/Hacia-donde-van-los-algoritmos-de-la-ADA-EASD-AACE-y-ALAD-en-el-tratamiento-DM2-Control-glucemico-y-proteccion-cardiovascular-como-objetivo-15-2048.jpg)
















![Oral
Pharmacologic
Treatment of
T2DM:
A Clinical
Practice
Guideline
Update from
the American
College of
Physicians
Qaseem A et al. Ann Intern
Med 2017 [Epub ahead of
print 3 January 2017]
doi: 10.7326/M16-1860
…monotherapy with metformin…
…add a second agent…
…add orals when lifestyle (has) failed…1
2
3](https://image.slidesharecdn.com/j03dmguidelines-171003141921/75/Hacia-donde-van-los-algoritmos-de-la-ADA-EASD-AACE-y-ALAD-en-el-tratamiento-DM2-Control-glucemico-y-proteccion-cardiovascular-como-objetivo-32-2048.jpg)