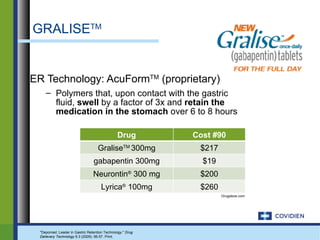
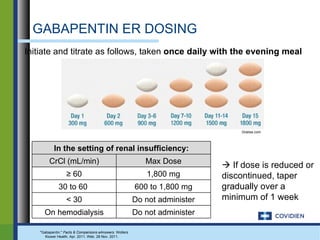
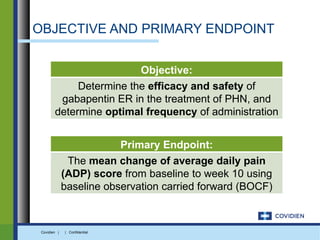
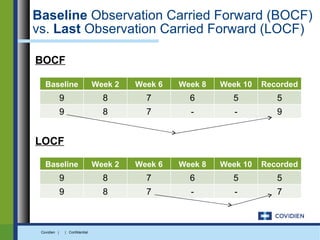
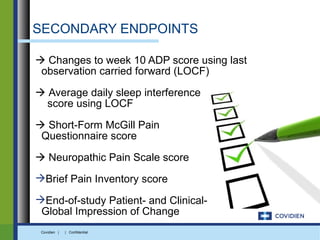
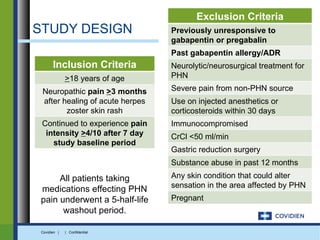
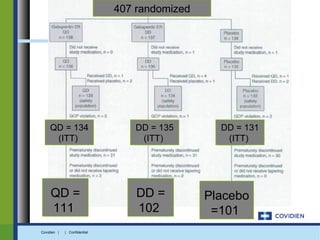
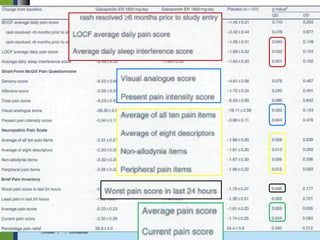
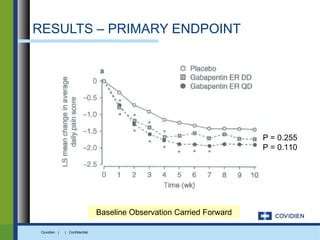
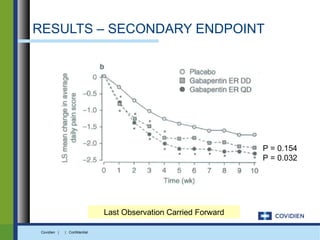
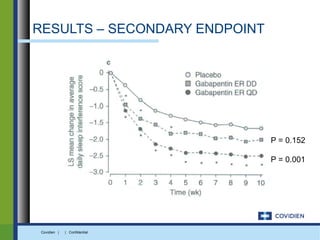
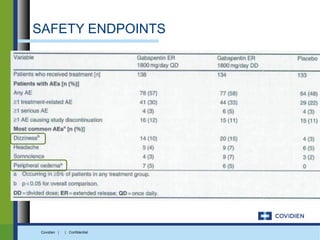
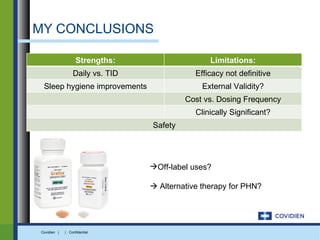
Extended-release gabapentin was approved in 2011 for the treatment of postherpetic neuralgia. A clinical trial compared once daily and twice daily dosing of extended-release gabapentin to placebo. While the primary endpoint was not statistically significant, secondary endpoints showed benefits for pain, sleep interference, and global impression of change with gabapentin. Further studies are needed to definitively establish efficacy and identify optimal dosing for postherpetic neuralgia.