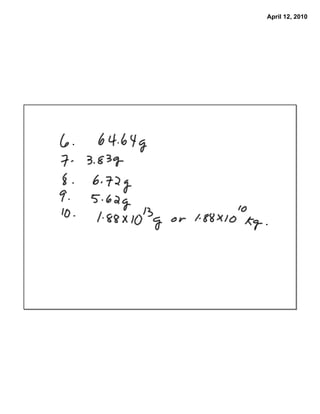Embed presentation
Download to read offline

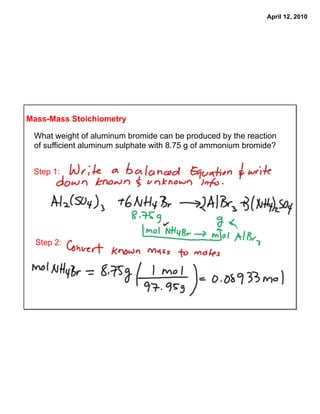


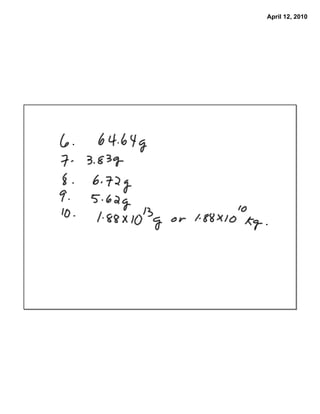
The document describes how to solve two stoichiometry problems. The first problem involves determining the mass of aluminum oxide produced from the reaction of 0.65 moles of oxygen gas with excess aluminum metal. The second problem involves calculating the mass of aluminum bromide that can be produced from the reaction of aluminum sulfate with 8.75 g of ammonium bromide. The document provides steps for solving each problem using mass-mass stoichiometry calculations.