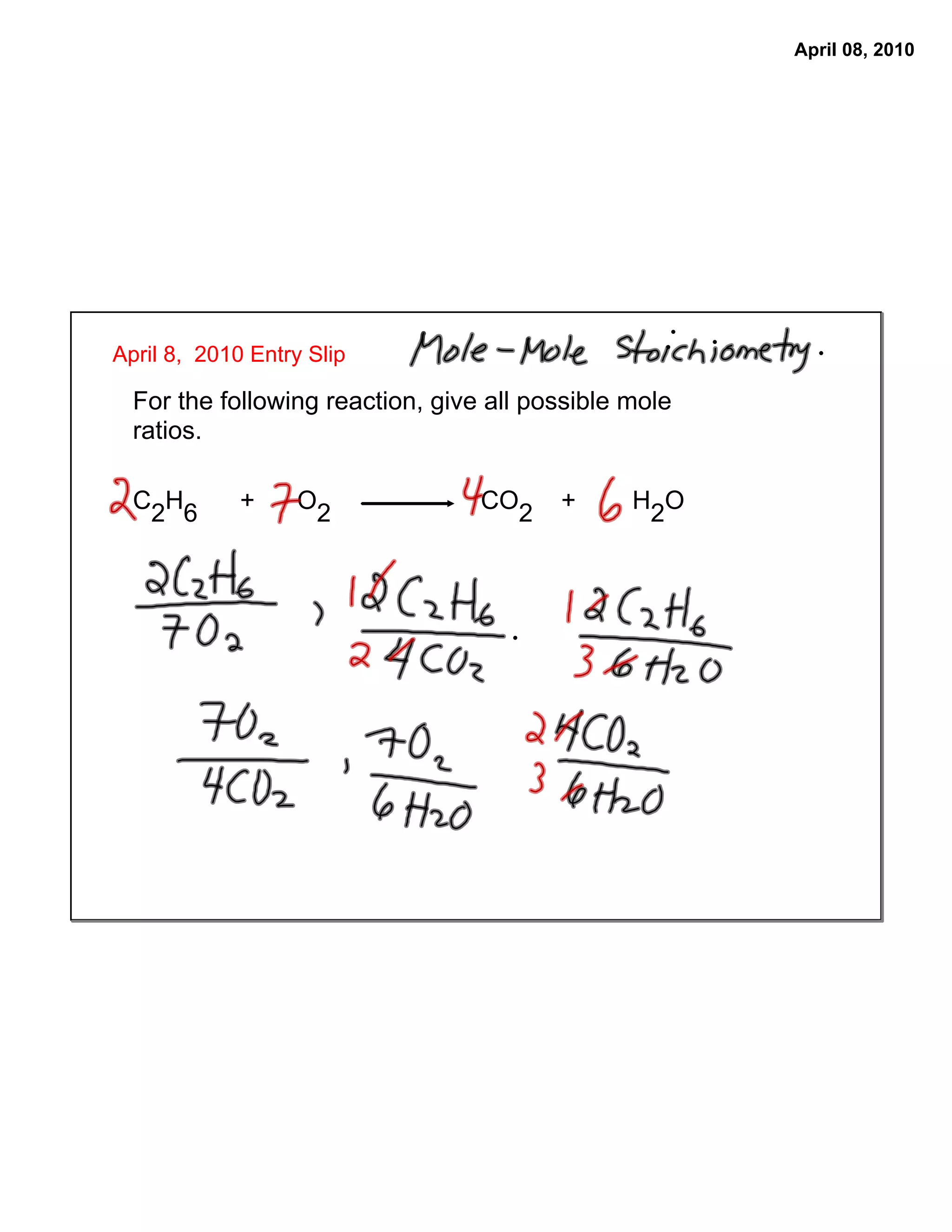
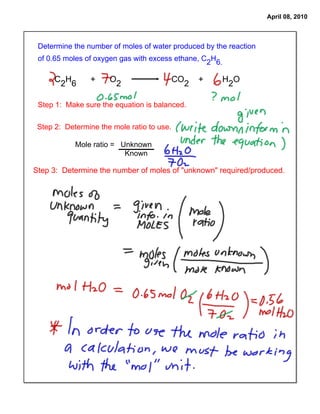
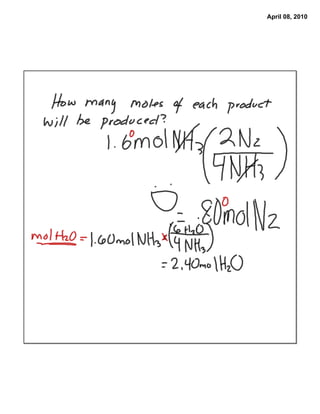
The document provides steps to determine the moles of water produced from a chemical reaction of ethane and oxygen gas. It states the balanced chemical equation and mole ratios can be used to determine the number of moles of an unknown reactant or product given the moles of a known substance. It then lists the steps as: 1) ensure the equation is balanced, 2) determine the mole ratio of unknown to known, and 3) calculate the moles of the unknown using the mole ratio and moles of the known.