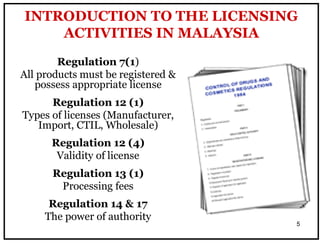
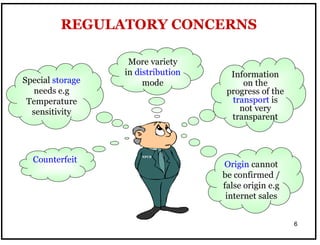
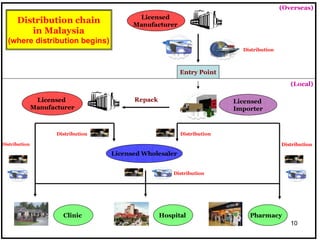
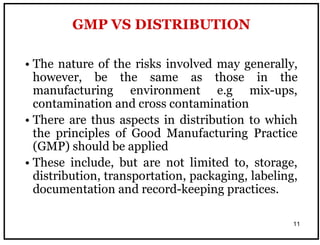
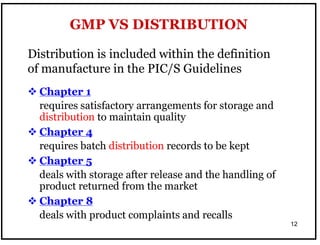
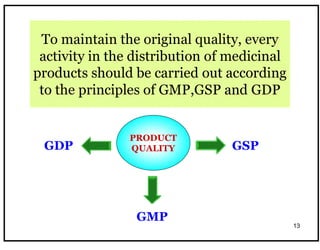
The document outlines the importance of Good Distribution Practice (GDP) in ensuring the quality of medicinal products throughout the distribution chain in Malaysia. It details the licensing requirements, regulatory concerns, and the need for strict adherence to principles of Good Manufacturing Practice (GMP), Good Storage Practice (GSP), and GDP to maintain product quality and safety. Additionally, the document addresses challenges faced in the industry, such as counterfeit products and the lack of harmonization of GDP standards.