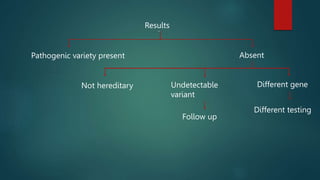
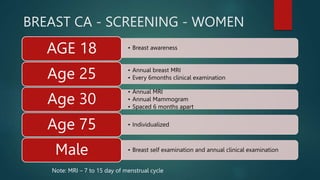
The document discusses the significance of genetic counseling, particularly in the context of hereditary cancer syndromes and BRCA mutations following a Supreme Court ruling against gene patents. It outlines the counseling process, including risk assessment and genetic testing, and highlights candidate profiles for counseling, management strategies for BRCA carriers, and emerging technologies like CRISPR for potential gene editing. Additionally, it emphasizes the evolving landscape of genetic testing and risk reduction strategies for cancer predisposition.






![Who is a candidate for cancer genetic
counselling ?
1. Early age of onset (e.g., younger than 50 y for breast, colon, and uterine
cancer)
2. Multiple family members on the same side of the pedigree with the same
cancer
3. Clustering of cancers/benign findings in the family known to be caused by
pathogenic variants in a single gene (e.g.,
breast/ovarian/pancreatic/prostate or metastatic]; colon/uterine/ovarian;
colon cancer/polyps/desmoid tumors/osteomas)
4. Multiple primary cancers in one individual (e.g., breast/ovarian cancer;
colon/uterine; synchronous/metachronous colon cancers; >15
gastrointestinal polyps; >10 adenomas; >5 hamartomatous or ≥3 juvenile](https://image.slidesharecdn.com/geneticscreening-240613160020-0a1b10a2/85/genetic-screening-in-cancer-presentation-7-320.jpg)
![6. Presence of a tumor that, by itself, indicates a need for genetics
evaluation
(e.g - male breast ca)
7. Pathology
(e.g., triple negative [ER/PR/Her-2] breast cancer 60 y and younger- BRCA
1; medullary thyroid cancer; a colon/endometrial cancer with an abnormal
microsatellite instability or immunohistochemistry result)
8. Tumor profiling results with possible germline implications (e.g.,
pathogenic BRCA1/BRCA2 variant detected by tumor profiling in any tumor
type)
9. Family history of a known pathogenic variant in a cancer predisposition
gene (e.g., BRCA1, MSH2, PTEN, CHEK2)](https://image.slidesharecdn.com/geneticscreening-240613160020-0a1b10a2/85/genetic-screening-in-cancer-presentation-8-320.jpg)














![BRCA screening
BRCA 1 – 50% Risk of Ca Breast
BRCA 2 – 85% RISK of Ca Breast
15-16% - Risk of ovarian Ca
Ovarian Ca – fallopian tube, primary peritoneal ca
Male ca breast, pancreatic cancer, melanoma [mainly BRCA 2]](https://image.slidesharecdn.com/geneticscreening-240613160020-0a1b10a2/85/genetic-screening-in-cancer-presentation-24-320.jpg)







![Future technologies - CRISPER
CRISPER – [clustered regularly interspaced short palindromic repeats]
CRISPR can “delete” and “replace” genes, which is referred to as
“editing the genome.”
Researchers are investigating whether this technology could be
used to inactivate genes and to incorporate new genes.
In theory, these discoveries may lead to fixes for defective
segments of genes, like those containing pathogenic variants, in
diseases such as BRCA-related breast and/or ovarian cancer
syndrome, cystic fibrosis, sickle cell anemia, and Tay-Sachs
disease.
Some laboratories have already reported successful trials treating
mice with genetic diseases by using this gene editing technology.](https://image.slidesharecdn.com/geneticscreening-240613160020-0a1b10a2/85/genetic-screening-in-cancer-presentation-33-320.jpg)